Thrombus formafigtion plays a significant role in disease of saphenous vein bypass grafts. Use of oral anticoagulants has not been tested in treatment of thrombotic occlusion of saphenous vein graft (SVG) disease. Here we describe the use of the novel oral anticoagulant rivaroxaban in the treatment of occlusive SVG disease with intraluminal thrombus, leading to successful recanalization.
A formação de trombos tem um papel significativo na doença do bypass do enxerto da veia safena (EVS). A utilização de anticoagulantes orais não foi testada no tratamento da oclusão trombótica na doença do EVS. Apresentamos o uso de um novo anticoagulante oral, o rivaroxabano, no tratamento da doença oclusiva do EVS com trombo intraluminal e recanalização bem-sucedida.
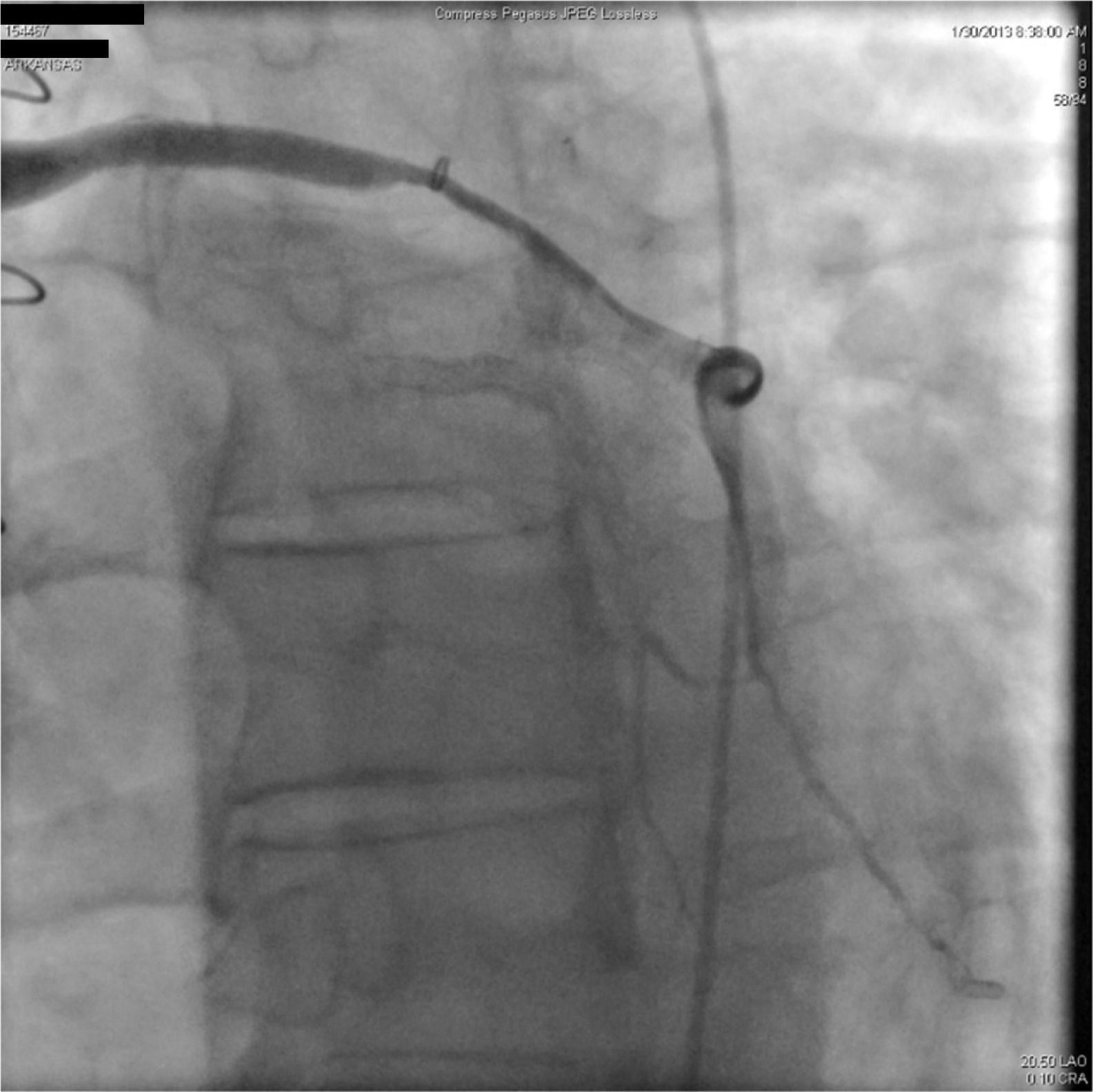
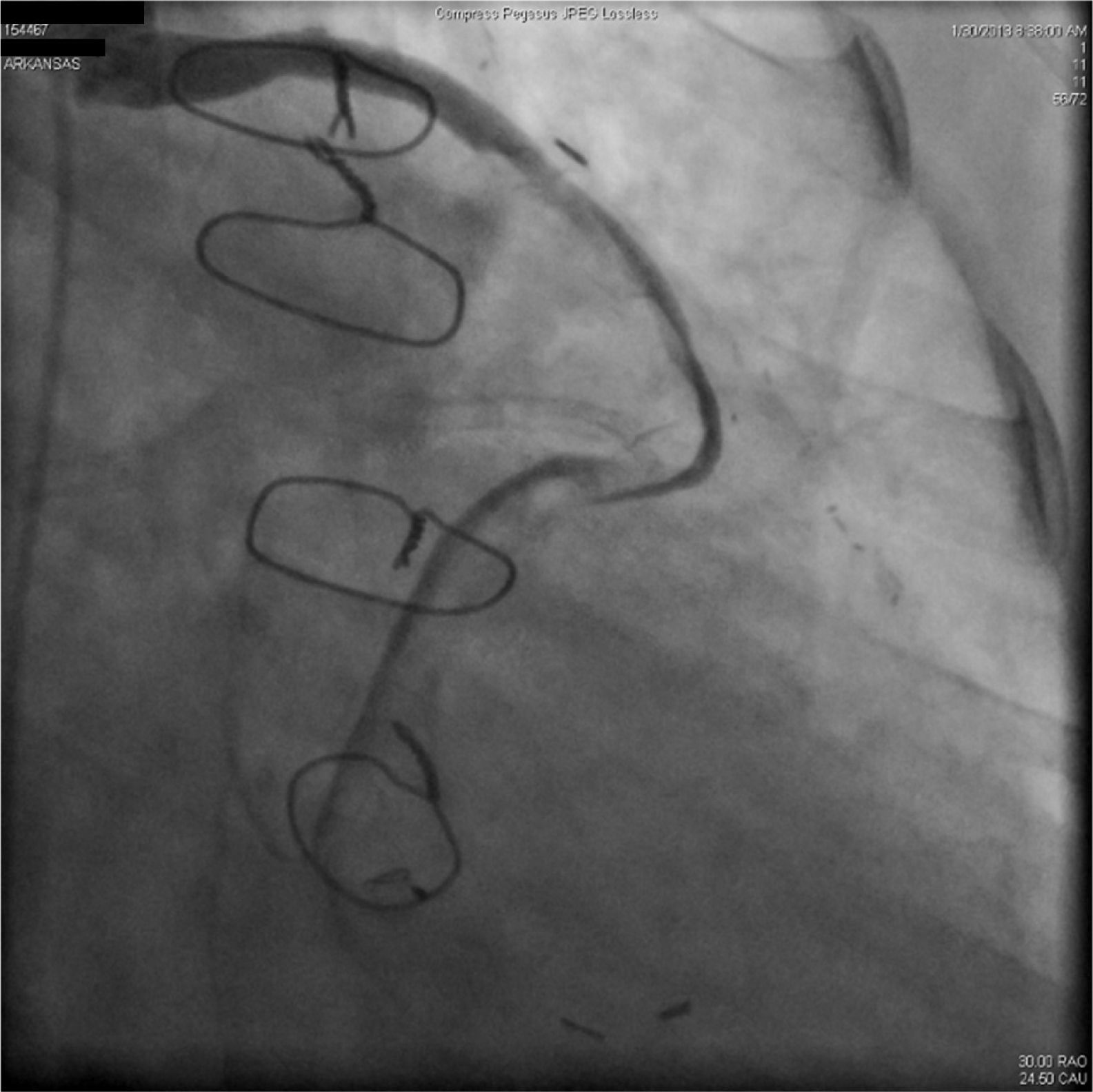
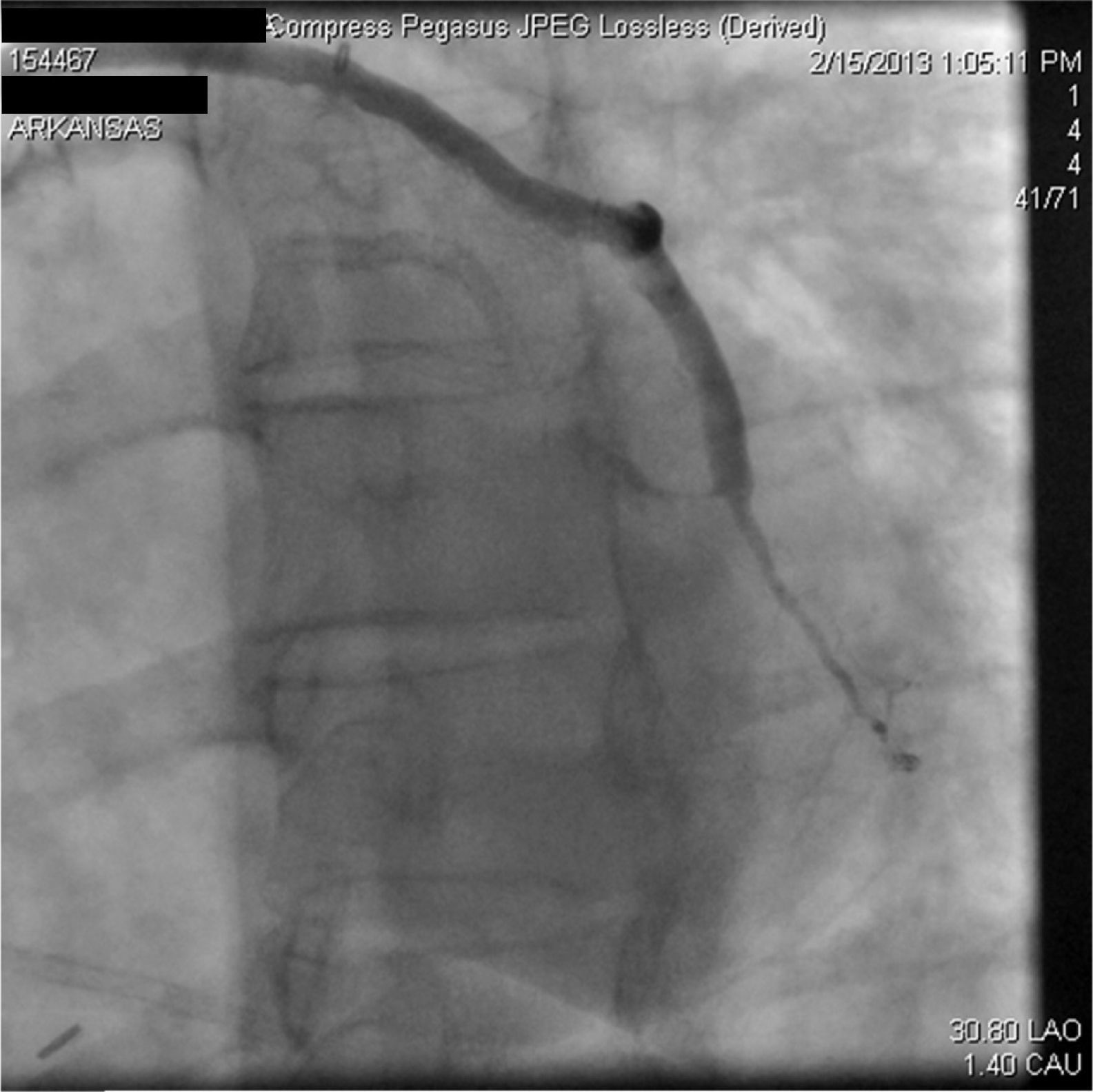
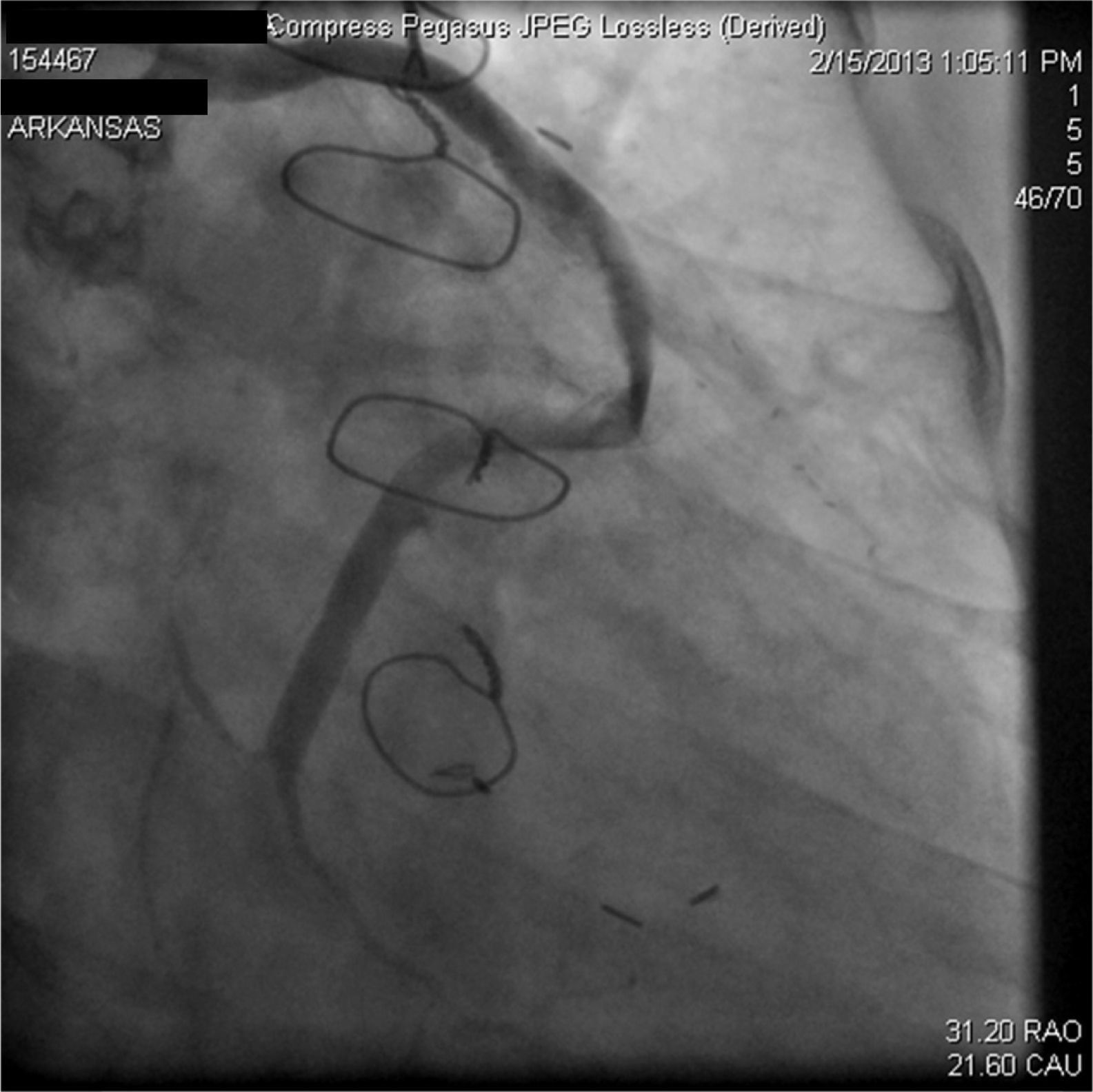
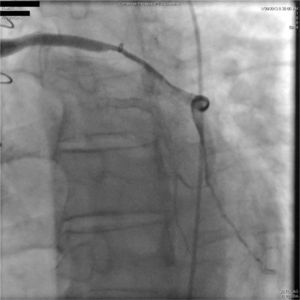
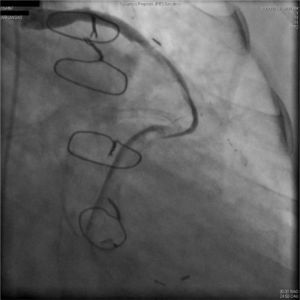
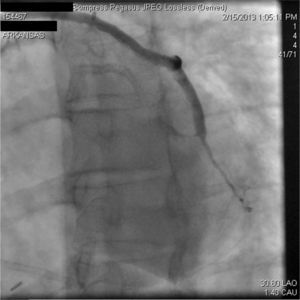
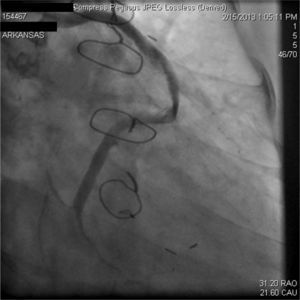
A 67-year-old woman with a history of coronary artery bypass grafting (CABG) six months previously underwent coronary angiography for stable class III angina. Extensive thrombus burden was visualized in the mid-distal segment of the saphenous vein graft (SVG) to the second obtuse marginal branch with decreased Thrombolysis In Myocardial Infarction (TIMI) 2 flow (Figures 1 and 2) and without evidence of obstructive atherosclerosis. The patient was placed on oral anticoagulation with rivaroxaban (Janssen Pharmaceuticals, Titusville, NJ, USA) 20 mg once daily. Follow-up catheterization after four weeks revealed angiographic resolution of the thrombus with significantly improved TIMI 3 flow (Figures 3 and 4).
Rivaroxaban is a new oral anticoagulant, a direct factor Xa inhibitor which is currently FDA approved for the prevention and treatment of venous thromboembolism, and for the prevention of stroke and systemic embolism in non-valvular atrial fibrillation.1 ATLAS ACS 2-TIMI 51 demonstrated reduced risk of the composite end point of death from cardiovascular causes, myocardial infarction, or stroke in patients with a recent acute coronary syndrome placed on rivaroxaban at the expense of increased risk of non-fatal major bleeding and intracranial hemorrhage.2 Thrombosis, intimal hyperplasia and atherosclerosis are the common mechanisms of SVG disease and occlusion; however, the Post CABG trial failed to demonstrate higher SVG primary patency rates with low-dose anticoagulation with warfarin as a primary prevention strategy.3 This case highlights the potential benefit of the new-generation oral anticoagulants in the secondary prevention and treatment of saphenous vein thrombosis-related disease. A similar approach was reported by Fiorina et al. in a small case series of 14 patients with non-ST-elevation myocardial infarction and occluded SVGs, in which after angioplasty with an undersized balloon to achieve TIMI flow 1-2, patients were placed on anticoagulation with warfarin for 11±7 days. A follow-up angiogram revealed that all the SVGs were patent, 14% of them with no residual lesions.4
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.