Pheochromocytoma is a tumor originating from chromaffin tissue. It commonly presents with symptoms and signs of catecholamine excess, such as hypertension, tachycardia, headache and sweating. Cardiovascular manifestations include catecholamine-induced cardiomyopathy, which may present as severe left ventricular dysfunction and congestive heart failure. We report a case of pheochromocytoma which was diagnosed following investigation of dilated cardiomyopathy. We highlight the dramatic symptomatic improvement and reversal of cardiomyopathy, with recovery of left ventricular function after treatment.
O feocromocitoma é um tumor originário do tecido cromafim. Habitualmente apresenta-se por sinais e sintomas de excesso de catecolaminas, tais como hipertensão arterial, taquicardia, cefaleias e sudorese. Das manifestações cardiovasculares inclui-se a miocardiopatia induzida por catecolaminas, que pode apresentar-se sob a forma de disfunção ventricular esquerda grave e insuficiência cardíaca congestiva. Relatamos o caso de um homem a quem foi diagnosticado feocromocitoma na investigação de um quadro de miocardiopatia dilatada. Realça-se neste doente a dramática reversão da miocardiopatia, com melhora sintomática e recuperação da função do ventrículo esquerdo após o tratamento.
blood pressure
computed tomography
electrocardiogram
emergency department
high frequency
low frequency
left ventricular
123I-metaiodobenzylguanidine
magnetic resonance imaging
standard deviation of R-R intervals
A 40-year-old man, apparently healthy until three months previously, had begun to suffer non-productive cough at night, weight loss (10–15 kg over three months), anorexia and dyspnea on progressively less exertion. The only relevant history was sporadic mild hypertension for several years for which he did not seek treatment, excessive sweating and persistently high heart rate (HR).
He consulted his family physician, who referred him to the pulmonary diagnostic center in his area of residence for suspected lung disease, including tuberculosis or atypical pneumonia. Diagnostic exams were inconclusive and various antibiotics were prescribed (amoxicillin/clavulanic acid, clarithromycin and levofloxacin) but he did not respond; one episode of fever (axillary temperature 39.0°C) was documented under antibiotic therapy. The patient's symptoms persisted and he began to suffer paroxysmal nocturnal dyspnea, orthopnea, pallor, constricting chest pain and profuse sweating, which prompted him to go to the emergency department (ED) and he was subsequently hospitalized.
On physical examination, the patient was anxious, apyretic but sweating, pallid, hydrated and not jaundiced. His blood pressure (BP) was 178/119 mmHg, HR 113 bpm, respiratory rate 20 cpm, peripheral oxygen saturation 92% with oxygen therapy through a nasal cannula at 2 l/min, with no jugular venous distension. Cardiopulmonary auscultation revealed tachycardia, no audible heart murmurs, and pulmonary rales in the lower half of both hemithoraces. Abdominal palpation showed no abnormalities and there were no signs of lower limb edema or deep vein thrombosis.
The electrocardiogram (ECG) showed sinus tachycardia, HR 104 bpm, and long QT interval (QTc 488 ms). The chest X-ray revealed bilateral interstitial infiltrate with obliteration of the right costophrenic angle.
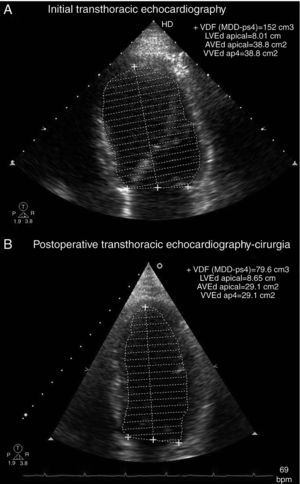
Transthoracic echocardiography showed left ventricular (LV) dilatation and global hypokinesia, with ejection fraction of 37%, and mild left atrial dilatation (Table 1 and Figure 1). NT-proBNP at admission was 7678 pg/ml.
Echocardiographic parameters before treatment and two months after surgery.
| Before treatment | Two months after surgery | |
| LVDD/BSA (cm/m2) | 3.3 | 2.8 |
| LVSD/BSA (cm/m2) | 2.7 | 2.0 |
| LVEDV/BSA (ml/m2) | 84 | 39 |
| LVESV/BSA (ml/m2) | 53 | 16 |
| LVEF (%) | 37 | 58 |
LVDD/BSA: left ventricular diastolic diameter indexed to body surface area; LVEDV/BSA: left ventricular end-diastolic volume indexed to body surface area; LVEF: left ventricular ejection fraction; LVESV/BSA: left ventricular end-systolic volume indexed to body surface area; LVSD/BSA: left ventricular systolic diameter indexed to body surface area.
Following heart failure therapy with angiotensin-converting enzyme inhibitors, diuretics and intravenous nitrates, there was a slight clinical improvement, particularly in dyspnea, orthopnea and paroxysmal nocturnal dyspnea. However, it did not result in control of BP or HR, and the patient remained hypertensive with sinus tachycardia.
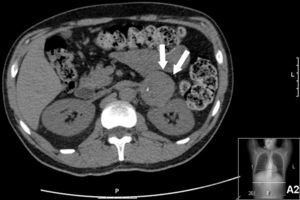
Thoracic-abdominal computed tomography (CT) showed mild apical fibrosclerosis, diffuse ground-glass infiltrates, with interlobular septal thickening, subcarinal adenopathy 1.5 cm in diameter, bilateral hilar enlargement, bilateral pleural effusion and a solid hypodense mass on the left adrenal gland, calcified in its interior and measuring 4.5×5.5 cm, with relatively well-defined borders and slight streaky densification of the adjacent fat (Figure 2).
Given the suspicion of pheochromocytoma, laboratory tests of plasma and urine catecholamines and their metabolites were performed, which showed elevation of all plasma catecholamines; 24-hour urine showed increased normetanephrine and adrenaline, but normal metanephrine (Table 2).
Initial assessment of plasma and urine catecholamines and their metabolites.
| Reference value | ||
| Plasma catecholamines | ||
| Adrenaline (pg/ml) | 160.2 | <100 |
| Noradrenaline (pg/ml) | 10 058.0 | <600 |
| Dopamine (pg/ml) | 827.7 | <100 |
| Total catecholamines | 11 045.9 | <800 |
| Urine catecholamines | ||
| Adrenaline (μg/24 h) | Undetectable | |
| Noradrenaline (μg/24 h) | 1943.9 | 12.1–85.5 |
| Dopamine (μg/24 h) | 187.8 | 0–498 |
| Normetanephrine (μg/24 h) | 6002.9 | 105–354 |
| Metanephrine (μg/24 h) | 180.6 | 74–297 |
| Vanillylmandelic acid (mg/24 h) | 24.9 | 1.8–6.7 |
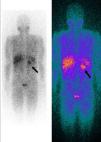
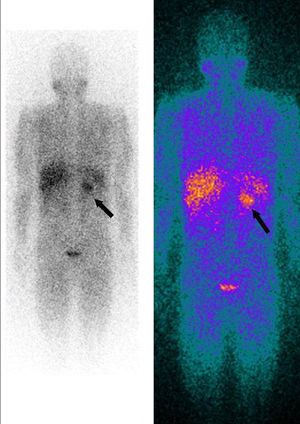
Whole-body 123I-metaiodobenzylguanidine (MIBG) scintigraphy revealed a nodular mass 3–4 cm in diameter at the same site on the adrenal gland as observed on abdominal CT, consistent with a diagnosis of pheochromocytoma. No other areas of abnormal uptake were detected (Figure 3).
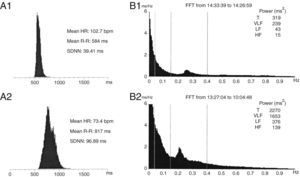
Twenty-four-hour Holter ECG monitoring (Figure 4) showed sinus rhythm, mean HR 103 bpm (74–123 bpm), with significantly diminished variability (standard deviation of R-R intervals [SDNN] 39 ms). Frequency analysis of HR variability showed that both high- (HF) (15 ms2) and low-frequency (LF) (43 ms2) components were virtually abolished.
(A) Histogram of RR intervals on 24-hour Holter ECG: (A1) initial and (A2) after surgery; (B) frequency analysis of heart rate variability: (B1) initial and (B2) after surgery. HF: high frequency; HR: heart rate; LF: low frequency; SDNN: standard deviation of R-R intervals; T: total; VLF: very low frequency. SDNN rose from 39 ms to 97 ms between the two exams. The HF (15 ms2) and LF (43 ms2) components were virtually abolished on initial assessment, and recovered after surgery (HF: 139 ms2, LF: 376 ms2).
Ambulatory BP monitoring revealed elevated 24-hour mean systolic and diastolic BP (systolic: 143 mmHg; diastolic: 92 mmHg), with a non-dipper pattern (nighttime fall 7%).
Preoperative management was begun with fluids and alpha- and beta-adrenergic receptor blockers, phenoxybenzamine being titrated up to 90 mg/day and bisoprolol (5 mg) added subsequently, resulting in BP control and a marked reduction in diaphoresis.
The patient underwent left laparoscopic adrenalectomy, with no significant hemodynamic alterations during the procedure. A 7 cm×4 cm×3.7 cm nodular mass, covered with adipose tissue 0.4–1.3 cm thick, was removed. Anatomopathological study of the specimen confirmed it to be a pheochromocytoma. Although the surgical procedure had been uneventful, the patient developed hypotension in the postoperative period, which was reversed with intravenous crystalloids. He was discharged medicated with furosemide 40 mg/day, spironolactone 25 mg/day, carvedilol 18.75 mg/day and enalapril 2.5 mg/day.
There was no biochemical or radiological evidence of pheochromocytoma during follow-up. Laboratory tests showed normalization of serum NT-proBNP to <20 pg/ml; 24-hour Holter ECG monitoring two weeks after surgery (Figure 4) showed sinus rhythm, significantly lower mean HR (73 bpm) than the previous exam and increased HR variability (SDNN 97 ms). Frequency analysis of HR variability revealed recovery of HF (139 ms2) and LF (376 ms2) components. Transthoracic echocardiography two months after surgery showed normalization of LV dimensions and recovery of LV systolic function (Table 1 and Figure 3). In view of the reversibility of the cardiomyopathy and the fact that the patient was asymptomatic, all medication was discontinued during follow-up.
DiscussionPheochromocytomas are catecholamine-secreting neuroectodermal tumors, most commonly arising from the adrenal medulla; extra-adrenal pheochromocytomas are known as paragangliomas. The incidence of pheochromocytoma is approximately 1–8/million in the general population, 0.1% in the elderly and 0.1–0.6% in hypertensive patients; 4–6.5% of adrenal ‘incidentalomas’ are pheochromocytomas. Pheochromocytomas can be sporadic or familial; sporadic forms are usually diagnosed between the ages of 40 and 50, whereas the hereditary forms, including those associated with multiple endocrine neoplasia type 2, neurofibromatosis type 1 and von Hippel-Lindau syndrome, present in childhood or early adulthood; most cases require genetic testing. Pheochromocytomas can be isolated or multiple and are usually benign, although there are malignant forms.1,2
The previously high mortality of pheochromocytomas (25–40%) has been reduced to <2%, primarily due to understanding of catecholamine physiology and adequate preoperative preparation.1
Hypertension, tachycardia, pallor, headache and anxiety usually dominate the clinical presentation, although some patients are asymptomatic. It has been suggested that the absence of hypertension in some patients with pheochromocytoma is due to predominant secretion of adrenaline, inactivation of noradrenaline inside the tumor, or tolerance of tissue receptors to circulating catecholamines.3 Both types of plasma catecholamines were elevated in our patient, but noradrenaline more markedly so.
Pheochromocytomas are associated with various cardiovascular complications, including LV hypertrophy, ischemic heart disease, myocardial infarction, cardiac arrhythmias, takotsubo cardiomyopathy, heart failure due to dilated cardiomyopathy and shock. The serious and potentially fatal cardiovascular complications of these tumors are due to the potent effects of catecholamines, especially noradrenaline, the main transmitter released from sympathetic nerve terminals.4
Patients with pheochromocytoma may present various disturbances in rhythm, conduction and ventricular repolarization on the ECG. Our patient had sinus tachycardia and long QT interval. Paroxysmal ventricular repolarization alterations, consisting of marked QT interval prolongation and inverted T waves, are frequently observed in patients with pheochromocytoma and can cause malignant ventricular arrhythmias.4,5
Assessment of HR variability is a noninvasive way of obtaining information on autonomic nervous system function in patients with pheochromocytoma. SDNN is one parameter that reflects autonomic activity. The low SDNN and the fact that the HF and LF components were virtually abolished in our patient are explained by inhibition of the central sympathetic nervous system by prolonged elevation of plasma catecholamines.6
Patients with pheochromocytoma usually have normal or increased ventricular systolic function on echocardiography, with only around 10% presenting catecholamine-induced cardiomyopathy,7 the echocardiographic features of which were first described in the late 1980s.8 Among the mechanisms suggested for transient LV ballooning are microvascular dysfunction of the coronary arteries, multivessel epicardial spasm, impaired fatty acid metabolism, myocarditis and catecholamine-mediated myocardial dysfunction.7 Various cases have been reported in which the first manifestation of pheochromocytoma was Takotsubo syndrome, the wall motion abnormalities being reversed after resection of the tumor; it is therefore recommended that pheochromocytoma should be ruled out in patients presenting with Takotsubo cardiomyopathy.9
The prognosis of catecholamine-induced cardiomyopathy associated with pheochromocytoma depends on early diagnosis and prompt medical and surgical treatment. Diagnostic exams in suspected pheochromocytoma include measurement of urine and plasma catecholamines and urine metanephrines (normetanephrine and metanephrine). However, since catecholamine release is often paroxysmal, a single measurement may not be sufficient to give a true picture; sensitivity can be improved by repeating the analysis two or more times, especially following a paroxysmal episode.
Preoperative evaluation to determine the location of a pheochromocytoma can be performed by a variety of imaging techniques, including CT, magnetic resonance imaging (MRI) and 123I-MIBG scintigraphy. CT has sensitivity and specificity of over 90% in locating adrenal pheochromocytomas, but is less accurate in detecting tumors in other locations. T2-weighted MRI images, with 91–100% sensitivity and 50–97% specificity, provide excellent anatomical detail and are better than CT at detecting paragangliomas. The radiopharmaceutical 123I-MIBG is structurally similar to noradrenaline and is thus selectively taken up and concentrated in chromaffin tissue. Its sensitivity of 77–91% and specificity of 96–100% makes it the method of choice to locate extra-adrenal tumors that are not detected on CT or MRI.10
Elective surgery is the treatment of choice for pheochromocytoma, and should be preceded by appropriate medical therapy for 10–14 days. However, in extreme conditions (e.g. shock due to hemorrhagic necrosis or rupture of a pheochromocytoma), when hemodynamic stabilization and adequate medical pretreatment are not possible, emergency tumor resection may be the only option.11
Preoperative drug therapy and appropriate intravascular volume expansion are key factors in improving prognosis in pheochromocytoma, and have reduced perioperative mortality to less than 2%. The usual strategy includes initial blockade of alpha-adrenergic receptors, for which phenoxybenzamine is most commonly used since it noncompetitively blocks alpha-adrenergic receptors. Beta-blockers are added after the first few days; these are particularly important in catecholamine-induced tachyarrhythmias, but should never be administered in the absence of effective alpha-adrenergic blockade since they can worsen hypertensive episodes by exacerbating vasoconstriction through inhibition of vasodilation mediated by beta-2 adrenergic receptors. Cardioselective beta-blockers are now preferred, since they act mainly on beta-1 adrenergic receptors.10
Laparoscopic adrenalectomy has become the standard approach for benign adrenal tumors, since it is associated with lower postoperative mortality, shorter hospital stay and lower cost than laparotomy. A recent study demonstrated that laparoscopic resection of pheochromocytomas measuring ≥6 cm in patients with no radiological evidence of malignancy is feasible and safe, with comparable results to resection of smaller tumors. There were no significant differences in intraoperative complications, estimated blood loss, cancer diagnosis, or recurrence; length of hospital stay was comparable and there were no incidents of capsular invasion or adverse cardiovascular events.12
Patients need to be monitored closely in the first 24 hours after surgery. The two main postoperative complications are hypotension and hypoglycemia. The first is due to a sudden drop in circulating catecholamines following tumor resection in the presence of continued alpha-adrenergic receptor blockade, as occurred in the case presented. The risk of hypoglycemia is related to hyperinsulinemia following recovery of insulin release after removal of the tumor.
Patients who present with acute heart failure may have worse prognosis as a result of extensive focal myocardial lesions. Nevertheless, catecholamine-induced cardiomyopathy due to pheochromocytoma has been shown to be reversible after surgical resection of the tumor.13,14
ConclusionsThe need for rapid differential diagnosis in a young patient presenting with congestive heart failure due to severe dilated cardiomyopathy prompted laboratory and imaging exams that revealed a pheochromocytoma.
Preoperative medical management and laparoscopic tumor resection were successful in achieving complete reversal of the clinical setting within a few weeks, with progressive normalization of LV and autonomic nervous system function, as shown by recovery of HR variability on assessment by 24-hour Holter ECG. We highlight the importance of including pheochromocytoma in differential diagnosis of unexplained cardiomyopathy in a young patient with signs and symptoms of catecholamine excess.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Satendra M, de Jesus C, Bordalo e Sá AL, et al. Reversibilidade da miocardiopatia induzida por catecolaminas associada ao feocromocitoma. Rev Port Cardiol. 2014;33:177.e1–177.e6.






 ECG: (A1) initial and (A2) after surgery; (B) frequency analysis of heart rate variability: (B1) initial and (B2) after surgery. HF: high frequency; HR: heart rate; LF: low frequency;
ECG: (A1) initial and (A2) after surgery; (B) frequency analysis of heart rate variability: (B1) initial and (B2) after surgery. HF: high frequency; HR: heart rate; LF: low frequency; 

