Dilated cardiomyopathy is the commonest form of cardiomyopathy in pediatric patients. Various causal factors have been identified, including ionic imbalance. Calcium ions play an essential role in regulating myocardial contractile function, and the harmful role of hypocalcemia as a coadjuvant or even precipitating factor of worsening heart failure has been described in rare case reports. Multiple causative factors may occasionally be present. We describe the first case, to our knowledge, of dilated cardiomyopathy in an infant with severe hypocalcemia and viral myocarditis.
A cardiomiopatia dilatada é a forma mais comum de cardiomiopatia em idades pediátricas. Várias causas podem estar envolvidas, incluindo desiquilíbrios iónicos. O cálcio desempenha um papel essencial na regulação da função contrátil do miocárdio. O efeito nocivo da hipocalcemia como coadjuvante, ou mesmo, fator precipitante do agravamento da insuficiência cardíaca tem sido episodicamente relatado. Raramente, múltiplas causas estão presentes no mesmo doente; descrevemos o primeiro caso, tanto quanto é do nosso conhecimento, de cardiomiopatia dilatada numa criança com hipocalcémia grave associada a miocardite viral.
Dilated cardiomyopathy (DCM), the commonest form of cardiomyopathy, has an estimated incidence of 1.13 cases per 100 000 children. It may be idiopathic, the product of familial genetic mutations, or the outcome of aggression to the myocardium caused by various factors, including metabolic or endocrine disturbances, electrolyte imbalance, inflammation, infection, immune system impairment and toxins. The primary etiology is diagnosed in fewer than half of these children, but this significantly improves their outcome. In isolated case reports hypocalcemia is a rare and reversible cause of heart failure or DCM.1–5 Multiple causative factors may occasionally be present. We describe the first case, to our knowledge, of DCM in an infant with severe hypocalcemia and viral myocarditis.
Case reportWe report the case of a three-month-old African boy, born in Europe, presenting with poor feeding, respiratory distress and irritability. These symptoms had progressively worsened over a period of three days. The patient was born at 40 weeks of gestational age with a birth weight of 3380 g. He had been exclusively breast-fed, with no vitamin D supplementation. Medical and family histories were irrelevant except for a maternal upper respiratory infection seven days before admission.
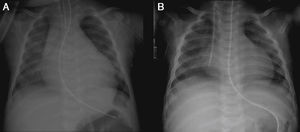
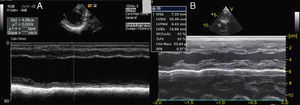
On admission he was apyretic, with pulse 190 bpm, respiratory rate 70–80 breaths per minute and oxygen saturation 95% in room air. His body weight was 6 kg (50th percentile). Physical examination revealed an infant of normal appearance but irritable and in respiratory distress. He was tachycardic, with an S3/S4 gallop rhythm. There were moist rales in the lung fields and chest wall retraction. The abdominal exam revealed hepatomegaly. The extremities displayed cyanosis and poor perfusion, with no edema. The chest X-ray showed cardiomegaly (cardiothoracic ratio 0.8) with pulmonary congestion (Figure 1A). The echocardiogram revealed a markedly enlarged left ventricular (LV) cavity with hypokinetic ventricular wall motion. LV end-diastolic diameter was 45 mm, LV end-systolic diameter was 40 mm, and fractional shortening (FS) was 5%, with no structural abnormalities (Figure 2). Electrocardiography showed sinus tachycardia (heart rate 190 bpm), LV hypertrophy and normal QTc.
Due to the extent of congestive heart failure, with poor systolic function, the patient was admitted to the cardiac intensive care unit, sedated and intubated. Arterial blood gas analysis revealed pH 7.27, pCO2 45.7 mmHg and HCO3 19 mmol/l under 50% FiO2 mechanical ventilation. Laboratory tests showed Ca2++ 0.76, lactate 11, total serum calcium 5.6 mg/dl (reference value: 8.8–10.8 mg/dl), inorganic magnesium 1.4 mg/dl (reference value: 1.8–2.6 mg/dl) and elevated alkaline phosphatase (422 IU/l, reference value: 82–383 IU/l), creatine kinase 497 IU/l (reference value <171 IU/l) and troponin I 0.43 ng/ml (reference value <0.06 ng/ml). Other biochemical parameters and electrolytes studied were within normal ranges. Enterovirus antigen was documented in feces.
Intravenous hyperimmune gamma globulin was given at admission and the patient was placed on milrinone and dopamine (titrated to a maximum dose of 0.48 μg/kg/min and 4 μg/kg/min, respectively) to improve systolic function, and furosemide for congestive heart failure. Two levosimendan cycles (0.2 μg/kg/min) were administered, at day 3 and 17 of disease.
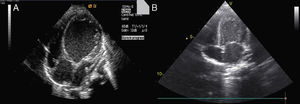
Decreased serum calcium levels remained an issue while in the intensive care unit, requiring calcium boluses and drips to improve levels, which led us to study the patient's phosphorus–calcium metabolism. Hormone levels that were changed included elevated parathyroid hormone (231.8 pg/ml, reference value: 12–80 pg/ml), decreased 25-hydroxyvitamin D (5.94 ng/ml, reference value 11–70 ng/ml) and decreased 1,25 (OH)2 vitamin D3 (18.57 pg/ml, reference value: 20.2–46.2 pg/ml). There were no metabolic disorders on amino acid and organic acid tests. Calcium and vitamin D deficiency was confirmed and replacement with calcium and alfacalcidol (0.05 μg/kg/day) was started. As the levels of serum calcium increased to normal, the patient showed rapid recovery of cardiac function, normalization of LV dimensions and function on echocardiogram (Figure 3) and reduction of cardiomegaly on chest X-ray (Figure 1B). As the infant improved clinically, he was weaned to oral captopril, furosemide, spironolactone and digoxin. He was discharged on the 25th hospital day and followed in the outpatient clinic, with calcium gluconate and calcitriol replacement.
DiscussionDCM is characterized mainly by LV systolic dysfunction, with an associated increase in mass and volume. In more severe cases, affected individuals present with signs and symptoms of heart failure. Young children often have poor appetite and cachexia. Sinus tachycardia, gallop rhythm, jugular venous distention, pallor, cold hands and feet, hepatomegaly, and a murmur consistent with mitral regurgitation are common findings at physical examination. Additionally, peripheral edema and ascites are late signs in children.6
According to data from the US National Heart, Lung, and Blood Institute's Pediatric Cardiomyopathy Registry, causal subgroups for DCM are idiopathic (66%), myocarditis (16%), neuromuscular disorders (9%), familial DCM (5%), inborn metabolic errors (4%), or malformation syndromes (1%).5,7 The course of DCM leading to heart failure in children varies. In general, in pediatric age cardiomyopathy is usually associated with significant morbidity and mortality, with nearly 40% of symptomatic patients receiving a heart transplant or dying within five years of diagnosis.8 Survival with conventional treatment is 64% at five years.9
Secondary DCM has many causes that are systemic in nature. Infectious causes of LV dysfunction that are consistent with the DCM phenotype are common, including various viral, bacterial and fungal infections. The most common viral pathogens that cause the human disorder are parvovirus B19, adenovirus, coxsackievirus B and other enteroviruses, influenza A, human herpes virus 6, cytomegalovirus, Epstein–Barr virus, herpes simplex virus type 1, and hepatitis C virus.
In our patient at least two possible causes for DCM were found: enterovirus infection and hypocalcemia. The myocarditis etiology is of a presumptive nature, based on documentation of the enterovirus antigen in feces, as no myocardial biopsy or histological studies were performed. It is also impossible to be sure which was the more significant etiology, or if both played a major role, as the simultaneous treatment of both situations prevented a clearer definition of the etiology. We may speculate, however, that in this patient the most significant factor leading to DCM and heart failure was severe hypocalcemia secondary to hypovitaminosis D as, despite optimum medical therapy with milrinone, dopamine and levosimendan, stabilization of the cardiac situation only occurred after administration of high doses of calcium and vitamin D and correction of ionic and endocrine imbalances.
The harmful role of hypocalcemia as a coadjuvant or even precipitating factor of worsening heart failure would appear to be obvious. Calcium ions play an essential role in regulating myocardial contractile function. Within the heart, calcium is essential for the initiation of excitation-contraction coupling via influx through L-type calcium channels. Once it is released from the sarcoplasmic reticulum by ryanodine receptors, calcium determines contractility by mediating the tension developed between actin and myosin filaments via the troponin–tropomyosin complex. Decreased available calcium leads to diminished responses in both of these areas and decreased cardiac function.10 However, heart failure is rarely described as a complication of hypocalcemia; generally only sporadic cases are found in the literature and ventricular systolic dysfunction usually regresses when the hypocalcemia is corrected, although the dilated pattern may persist for a long time.2
Calcium metabolism is mainly regulated by parathyroid hormone and vitamin D, which affect calcium absorption and deposition in bones. Hypocalcemic rickets used to be a rare disease due to successful supplementation of breast-fed infants with vitamin D. However, the prevalence of rickets has increased in breast-fed infants over the last ten years.10 The reason for this re-emergence is unknown; there may be a lack of parental education on vitamin D supplementation.
Breast milk alone can meet nutrient needs during the first six months of life, except for vitamin D, vitamin K and iron. The vitamin D content of human milk has been estimated to range between 5 and 136 IU/l depending on the season. Studies in Greece, Italy and France report that subclinical vitamin D deficiency is not uncommon in Europe. Maternal vitamin D insufficiency during pregnancy leads to an inadequate supply to the embryo through the placenta, the main supplier of 25-hydroxyvitamin D. As a consequence, the neonate has deficient reserves. In addition, the exclusive breast-feeding of infants without vitamin D supplementation may lead to the development of nutritional rickets during the first year of life.11
There is also an increasing trend toward inadequate light exposure of susceptible dark-skinned infants, especially outside Africa, in urban settings and during the winter months.10,12 Other causes of vitamin D deficiency include malabsorption syndromes and renal disease.
Nutritional DCM and its reversal with prompt treatment involve mechanisms that remain to be fully elucidated. In our patient stabilization of the cardiac situation only occurred after administration of high doses of calcium and vitamin D and correction of ionic and endocrine imbalances.
ConclusionWith this case report we highlight the importance of screening for ionic imbalances when initially assessing a patient with DCM and the possibility that multiple causes may be present. It is also important to stress the need for vitamin D supplementation in breast-fed infants, not only for bone health, but also for cardiac function.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.