Libman-Sacks endocarditis (LSE) is the most characteristic cardiac manifestation of systemic lupus erythematosus (SLE). It is usually clinically silent but heart failure due to valvular dysfunction, secondary infective endocarditis and embolic phenomena can complicate valvular abnormalities. We present a patient with SLE and blindness due to right central retinal artery occlusion. Echocardiographic examination revealed a verrucous vegetation on the posterior mitral valve leaflet consistent with LSE. Anticoagulation therapy was started. Echocardiographic regression of the vegetation was observed and there has been no recurrence of thromboembolic events to date.
A endocardite de Libman-Sacks é a manifestação cardíaca mais característica do LES. É habitualmente clinicamente silenciosa, mas a insuficiência cardíaca por disfunção valvular, a endocardite infecciosa secundária e os fenómenos embólicos podem complicar as alterações valvulares. Apresentamos um caso clínico de uma doente com LES e amaurose à direita por trombose da artéria central da retina. O ecocardiograma mostrou uma vegetação verrucosa no folheto posterior da válvula mitral, compatível com endocardite de Libman-Sacks. A doente iniciou terapêutica com anticoagulação, verificou-se regressão da vegetação descrita e não teve recorrência de eventos tromboembólicos até à data.
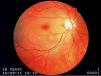
A 73-year-old woman with a history of systemic lupus erythematosus (SLE) diagnosed 15 years ago, treated with corticosteroids and hydroxychloroquine, presented to an ophthalmology consultation after a three-hour period of sudden and marked decreased right visual acuity. No other symptoms were reported, including fever or localized weakness. Fundoscopic examination showed a pale retina with a cherry-red macula (Figure 1) and a diagnosis of right central retinal artery occlusion was made.
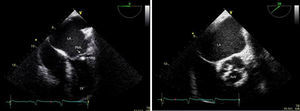
Transthoracic and transesophageal echocardiography revealed the presence of a mass attached to the ventricular side of the posterior mitral leaflet, with a vibratory motion, a maximum diameter of 13 mm, irregular shape and heterogeneous echogenicity, consistent with vegetation (Figure 2). The aortic valve had thickened leaflets (Figure 2) and moderate regurgitation by color Doppler.
Carotid Doppler ultrasound showed no significant atherosclerotic lesions and no other cardioembolic sources were detected.
Inflammatory parameters (white blood cell count 6.9×109/l, C-reactive protein 1.6 mg/dl and erythrocyte sedimentation rate 44 mm/h) were not suggestive of infection. Blood cultures were negative. Autoimmunity study revealed antinuclear antibodies positive at a titer of 1/320, with no other positive antibodies, including negative antiphospholipid antibodies (APA). C3 and C4 levels were normal. Thrombophilia tests including C and S protein levels, antithrombin III and resistance to activated protein C were also normal.
We assumed a diagnosis of Libman-Sacks endocarditis (LSE) and the patient started anticoagulation therapy. A follow-up transesophageal echocardiogram four weeks later showed resolution of the previously described vegetation. There was no recurrence of thromboembolic events.
DiscussionSLE is an autoimmune disease that causes multiorgan inflammatory damage. In recent decades, with increasing survival and advances in diagnostic techniques, particularly in echocardiography, cardiac disease associated with SLE has become more evident.
Valvular disease is one of the main cardiac manifestations of SLE and can occur in the form of valvular thickening, masses or noninfective vegetations (LSE), valvular regurgitation and valvular stenosis.1
LSE was first described in 1924 by Libman and Sacks in four patients with SLE and noninfective verrucous vegetations.2 Libman-Sacks vegetations develop mainly on the mitral valve, followed by the aortic valve, but may develop on any other valve, on the subvalvular apparatus or on the surface of the endocardium.1 They are usually located on the atrial side of the mitral valve leaflets or the vessel side of the aortic valve leaflets.3
A significant proportion of patients with SLE have LSE detected in autopsy studies (30–50%). However, the real prevalence of LSE remains unknown since most patients with Libman-Sacks vegetations have asymptomatic valve abnormalities.1 Moyssakis et al.1 studied 342 patients with SLE by echocardiography over four years and found an 11% incidence of LSE and an association with lupus duration, disease activity, presence of anticardiolipin antibody and manifestations of antiphospholipid syndrome. Roldan et al.3 studied 69 patients with SLE by transesophageal echocardiography and found a 43% incidence of LSE, which may be related to the greater accuracy of this modality.
It has been proposed that LSE is due to the formation of fibrin-platelet thrombi on the injured valve, followed by tissue organization and leading to valvular fibrosis, distortion and subsequent dysfunction. Recent studies have shown deposition of immunoglobulins and complement in the valvular structure which subsequently developed LSE and valvular thickening.4
The association of LSE and APA has been widely investigated and has been reported in several studies,1,4,5 although others have found no connection.6 The role of APA in the pathogenesis of valvular disease is thought to be by promoting thrombus formation on injured valve endothelium and inflammatory changes, rather than a more direct pathogenic role.4 Further, the observation that there is a significantly higher prevalence of valvular lesions in patients with antiphospholipid syndrome (APS) secondary to SLE than in those with primary APS may mean that there are SLE-related factors that promote endocardial damage and contribute to this difference.1,4 APA were not detected in our patient, which is in agreement with this theory.
One recognized complication of LSE is the development of secondary infectious endocarditis3,4 which increases the complexity of differential diagnosis in a patient with SLE who presents with a valve mass. Infective endocarditis lesions are usually located at the leaflet's line of closure, are homogeneous in echogenicity and may show a vibratory or rotatory motion.4 In contrast, LSE lesions are usually located at the base, middle or tip of the leaflets and are variable in shape and size and heterogeneous in echogenicity.3 Thus it is imperative to differentiate between these two clinical identities, since management and treatment are quite different. Our patient had no fever, her leukocyte count was normal and blood cultures were negative, which enabled us to reach a diagnosis of LSE and to initiate anticoagulation therapy. Follow-up transesophageal echocardiography provided an assessment of disease progression, revealing the regression of the previously observed vegetation.
The main clinical impact of LSE is related to the probability of lesion progression to valvular dysfunction and the tendency to thromboembolic events, especially stroke or transient ischemic attack.1,3,4 The incidence of thromboembolic cerebrovascular events in patients with LSE has been reported as 10–20%1,3 and a cardioembolic origin was assumed in most cases. In our search of the literature we found several case reports of distal embolization from LSE, the majority reporting cerebral embolization, but none with retinal embolization.
In the present case, there was an occlusion of the right central retinal artery, which originates from the ophthalmic artery, the first intracranial branch of the internal carotid artery. Given the temporal relationship between the onset of right blindness and the echocardiographic finding of a mass adhering to the mitral valve, it was assumed that the retinal artery occlusion was cardioembolic in origin. The patient was started on anticoagulation therapy for secondary thromboprophylaxis and remission of the previously detected vegetation was achieved. To date she has had no new thromboembolic events.
With this case report we highlight the importance of awareness of this entity, allowing rapid referral for cardiovascular examination and thus enabling early diagnosis and appropriate intervention.
Given that most patients with SLE and valvular disease have no cardiac symptoms, a careful cardiovascular examination should be made periodically. Since strokes in patients with SLE are frequent and, on the other hand, valvular thickening and vegetations are common and can act as substrates for cardioembolism, prophylactic therapy with anticoagulation may be an appropriate approach to these patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.