Consumption of a Western diet during the perinatal period is associated with development of cardiovascular disease. Resistance training (RT) has been used to treat cardiovascular disorders. The aim of this work was to assess the effect of RT on cardiometabolic disorders in rats exposed to a Western diet in the perinatal period.
MethodsFemale Wistar rats were fed with control or Western diet during pregnancy and lactation. The pups were divided into three groups: Control (C), Western Diet Sedentary (WDS) and Western Diet+RT (WDRT). At 60 days of age, all animals started the RT protocol (five times a week for four weeks). At the end, blood pressure was recorded for analysis of heart rate variability and baroreflex sensitivity (BRS). Blood samples were collected for biochemical analysis.
ResultsRT reduced blood pressure and vascular sympathetic modulation and increased BRS. There were improvements in biochemical profile, with reductions in fasting blood glucose, total cholesterol and low-density lipoprotein, and an increase in high-density lipoprotein.
ConclusionRT led to beneficial adaptations in the cardiovascular system, mediated by changes in the mechanisms of autonomic control and biochemical profile of animals exposed to a Western diet in the perinatal period.
O consumo da dieta ocidental está associado ao surgimento de doenças cardiovasculares. O treinamento de resistência (TR) tem sido utilizado no tratamento destas doenças. O objetivo deste trabalho foi avaliar o efeito do TR sobre as alterações cardiometabólicas na prole de ratas expostas a dieta ocidental no período perinatal.
MétodosRatas Wistar receberam dieta controle ou ocidental durante a gravidez e lactação. Os filhotes foram divididos em três grupos: Controle (C), dieta ocidental sedentário (OCS) e dieta ocidental+TR (OCTR). Aos 60 dias de vida, os animais iniciaram o protocolo de TR realizado cinco vezes por semana durante quatro semanas. Ao fim, foi registrada pressão arterial para análise da variabilidade da frequência cardíaca e sensibilidade do barorreflexo (SBR). Amostras de sangue foram coletadas para análise bioquímica.
ResultadosO TR foi capaz de reduzir a pressão arterial, a modulação simpática vascular e aumentar a SBR. Houve melhoria no perfil bioquímico, com redução na glicemia de jejum, colesterol total e lipoproteínas de baixa densidade, além de aumento das lipoproteínas de alta densidade.
ConclusãoO TR promoveu adaptações benéficas ao sistema cardiovascular, mediadas por ajustes nos mecanismos de controle autonômico e perfil bioquímico dos animais expostos à dieta ocidental no período perinatal.
one-repetition maximum test
American College of Sports Medicine
blood pressure
baroreflex sensitivity
diastolic blood pressure
glucose transporter 4
Household Budget Survey
high-density lipoprotein
high frequency component
heart rate
low-density lipoprotein
low frequency component
mean arterial pressure
nucleus of the solitary tract
pulse interval
paraventricular nucleus of the hypothalamus
resistance training
systolic blood pressure
total cholesterol
triglycerides
very low-density lipoprotein
very low frequency component
Western diet trained
Western diet sedentary
Eating patterns in the modern world are changing, with increasing consumption of low-cost, high-calorie, processed foods high in sodium and saturated fat and low in other essential nutrients. This is known as the Western diet.1,2 At the same time, technological advances and modern lifestyles have reduced the need for people to move, resulting in increased sedentarism. These factors are leading to increases in overweight and obesity.3
Studies show that excessive consumption of nutrients in early life (intrauterine and/or pre- and post-natal stages) leads to morphological and functional changes in the fetus and to the development of cardiovascular disease in adulthood, in both animals4 and humans,5 a phenomenon known as fetal or metabolic programming.6,7
This process can alter phenotypic characteristics, leading to dysregulation of energy balances and to gains in body weight, predisposing the individual to obesity and associated comorbidities.8,9 In a previous study, we showed that animals fed a Western diet in the perinatal period had altered biochemical profiles and cardiovascular dysautonomia, followed by hypertension in adulthood.9
Resistance training (RT) is used as a way to improve physical and muscular fitness, increase lean mass and maintain basal metabolic rate. RT has also been shown to foster beneficial adaptations in cardiovascular function.10,11
We therefore hypothesized that the cardiovascular benefits of RT are brought about by adaptations in blood pressure (BP) control mechanisms and that it will improve the biochemical profile of rats exposed to a Western diet in the perinatal period. Accordingly, the study aimed to observe the effects of RT on cardiovascular and biochemical variables in these animals.
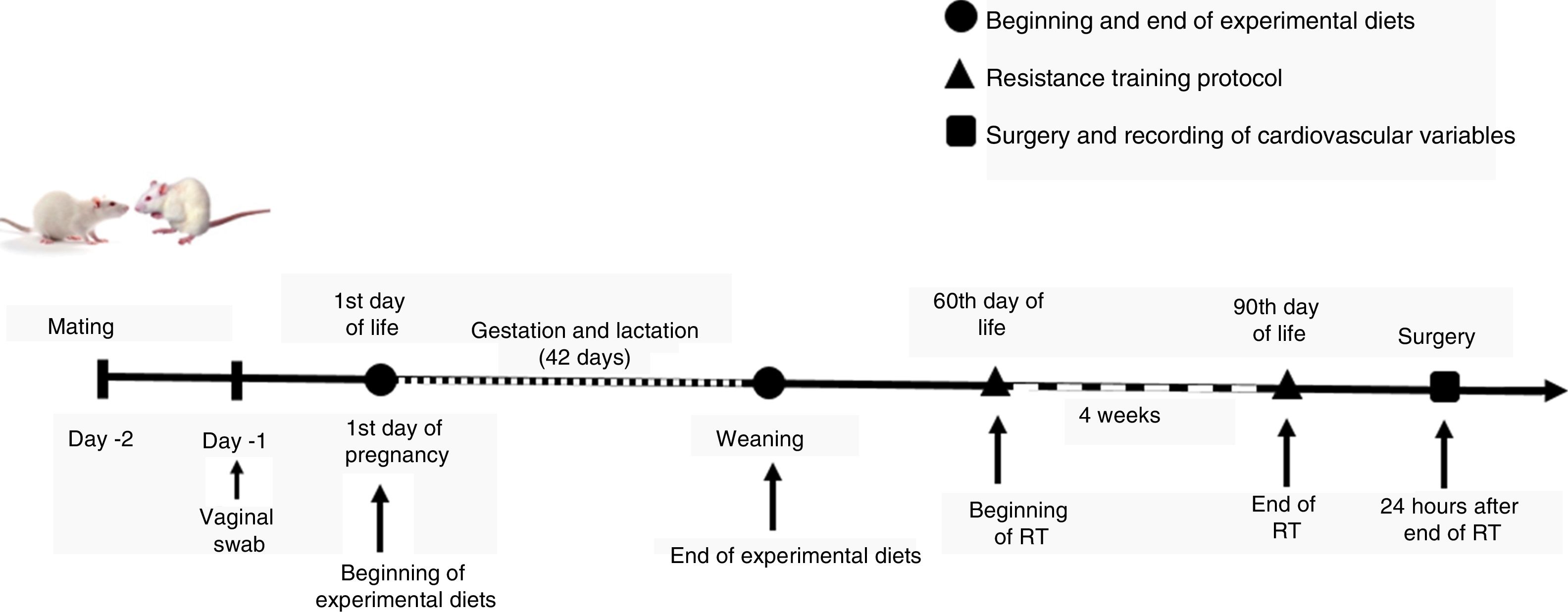
MethodsAnimalsThe present study meets the standards for conducting research on animals and its procedures followed the ethical principles of animal experimentation laid down by the Brazilian National Council for the Control of Animal Experimentation (CONCEA), based on the 2013 Brazilian guidelines for the care and use of animals for scientific and teaching purposes (DBCA). It was approved by the Committee on Animal Research and Ethics of the Federal University of Sergipe (CEPA/UFS), protocol 10/2014.
Wistar rats of both sexes were kept in polypropylene cages with water and chow ad libitum at a temperature of 22±1 °C. Fifteen virgin female Wistar rats, aged between 90 and 120 days and weighing 250-300 g, were mated with fertile males in the proportion of four females to one male. A vaginal swab was used daily to check for pregnancy from the presence of sperm. The female rats were then divided into two groups according to diet: Control (n=5) and Western (n=5), until weaning. The litters were each adjusted to eight neonates 24 hours after birth, maintaining the same proportion of males and females when possible. Only males were used in the experimental protocols, to prevent the hormonal fluctuations due to the estrous cycle from influencing the results.
After weaning, the pups were divided into three groups: Control (C, n=7), pups of mothers fed a control diet that did not perform RT; Western diet sedentary (WDS, n=7), pups of mothers fed with a Western diet that did not perform RT; and Western diet trained (WDRT, n=7), pups of mothers fed with a Western diet that performed RT.
After weaning, the animals were fed with Labina® commercial chow for rodents. According to the manufacturer (Purina do Brasil Ltda), the chow contains minimum 23% crude protein, minimum 4% volatile substances, maximum 10% ash and 5% fiber, and has an energy content of 3.6 kcal/g.
DietsThe control diet used during gestation and lactation was casein-based AIN-93G, specifically for growing rodents.12 The formulation of the experimental Western diet was based on the Brazilian Household Budget Survey (HBS), with some adjustments to the percentages of its composition. These included increased lipid and simple carbohydrate content in order to enhance organoleptic factors, to give the feed a similarly pleasant odor and texture to the Western diet. The protein content was also adjusted, since the proportion of protein in the HBS diet is inadequate for the reproduction and growth stages in rats, and a final adjustment was made for the adult rats’ maintenance stage (15.5% protein).
The Western diet contained 31% lipids, mainly saturated fats (67.59%), and 51.7% carbohydrates, mainly in the form of simple sugars, which resembles Western diets in humans, while the control diet contained 25.99% saturated fats and 55% carbohydrates.
The Control (AIN-93G) and Western diets were produced by the Laboratory of Experimental Nutrition and Diet (LNED) of the Department of Nutrition of the Federal University of Pernambuco and were stored at 4 °C until used(Figure 2).
Resistance training protocolRT was performed using a squat-training apparatus.13 The animals underwent a five-day adaptation period before training by being handled and placed in the device in the starting position of the exercise, with no added load.
After adaptation, all the animals underwent a one-repetition maximum test (1RM) to determine the loads to be used. In the 1RM, the animal fully extends its hind legs under the maximum possible load while completing the movement. The desired intensity can thus be calculated based on the loads raised in the test. The RT protocol started after 48 hours, and consisted of three series of 10 repetitions with an intensity of 40% of 1RM (defined as low intensity),14 with 1-min intervals between the series for recovery. Each RT session lasted approximately 5 min. RT was performed five times a week for four weeks, for a total of 20 RT sessions. A new 1RM was carried out every 15 days to adjust the loads.
The animals were stimulated to perform the exercise by electrodes placed on the tail (BIOSET, Physiotonus four, Model 3050, Rio Claro, São Paulo). The parameters used were frequency 1 Hz, pulse width 1 ms, on time 1-3 s, off time 2 s, and sufficient intensity for the animals to perform the exercise (4-15 mA). The animals in groups C and WDS were placed in the squat-training apparatus and electrostimulated with the same parameters as for those in the WDRT group, but the equipment was kept in the rest position and without added load.
Experimental designAssessment of cardiovascular functionThe animals were anesthetized with intraperitoneal (IP) sodium thiopental (50 mg/kg) and a polyethylene catheter (PE-10/PE-50, Intramedic, Becton Dickinson, Sparks, MD, USA) was surgically implanted in the left femoral artery. The catheter was exteriorized subcutaneously in the posterior cervical region. After surgery, the animals received 1.1 mg/kg IP of flunixin meglumine (Banamine®, Schering-Plough, Kenilworth, NJ, USA) to reduce hyperalgia and postoperative inflammation, for the animals’ well-being.
Twenty-four hours after the procedure and after recovery from the effects of the anesthesia, the catheter was connected to a pressure transducer coupled to a pre-amplifier (FE221, Bridge Amp, ADInstruments, Bella Vista, NSW, Australia) using the PowerLab recording system (ADInstruments, Bella Vista, NSW, Australia). BP was recorded for 30 min and processed using LabChart 7 software (ADInstruments, Bella Vista, NSW, Australia), in order to identify inflection points and to generate beat-by-beat time series. All readings were taken in the morning, in order to ensure that behavioral factors would influence all the animals equally. Mean arterial pressure (MAP), systolic BP (SBP), diastolic BP (DBP), pulse interval (PI) and heart rate (HR) were measured and analyzed.
Autonomic assessmentVariability in PI and SBP was analyzed using CardioSeries software, version 2.4 (http://sites.google.com/site/cardioseries), as described previously.15 Beat-by-beat series were obtained based on the BP recordings and converted into 100-ms points (10 Hz) by cubic spline interpolation. The data were divided into partially overlapping sequences and periods of 512 points (51.2 s). For calculation of spectral power, the data were inspected visually and non-stationary segments were discarded.
The spectrum was calculated directly by fast Fourier transform and a Hanning window was used to attenuate distortion. The spectrum was divided into low frequency (LF) (0.2-0.75 Hz) and high frequency (HF) (0.75-3 Hz) bands. The results were expressed in normalized units by dividing the LF and HF powers by the total power minus very low frequency (VLF) (<0.2 Hz) power. The LF component of PI represents cardiac sympathetic modulation, the HF component of PI is an index of vagal modulation, while the LF/HF ratio measures cardiac autonomic balance. The LF component of SBP represents vascular sympathetic modulation.
Spontaneous baroreflex sensitivity (BRS) was quantified by the sequence method. The CardioSeries software was used to process beat-by-beat time series for SBP and PI, searching for sequences of at least four consecutive beats in which increases in SBP were followed by lengthening of PI and decreases in SBP were followed by shortening of PI. Data with a linear correlation of >0.85 were considered to be baroreflex sequences. The slope of the linear regression lines between SBP and PI was used as an index of BRS.15
Biochemical assessmentThe animals were anesthetized with halothane after 12 hours fasting, and blood was collected from the retro-orbital plexus using heparinized capillary tubes. The sample was placed in a separation tube and centrifuged at 3500 rpm for 5 min to obtain plasma. The supernatant was drawn off using a pipette and transferred to an Eppendorf® tube, which was sent for biochemical analysis of glucose, total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL) and low-density lipoprotein (LDL).
A colorimetric enzyme assay (Bioclin, Belo Horizonte, Minas Gerais, Brazil) was used to measure plasma concentrations of the biochemical parameters, which were analyzed in an 800 XI spectrophotometer (Femto, São Paulo, Brazil), in accordance with the manufacturer's instructions. The Friedewald formula was used to measure LDL and very low density lipoprotein (VLDL).16
Statistical analysisValues were reported as mean ± standard error of the mean. The results for hemodynamic assessment, autonomic modulation and biochemical profile were analyzed using one-way analysis of variance followed by the Bonferroni post hoc test to assess differences between means in the two groups. Values of p<0.05 were considered statistically significant. GraphPad software version 5.0 (San Diego, CA, USA) was used for the statistical analysis.
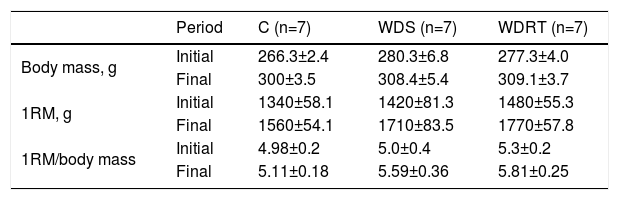
ResultsChanges in body weight and one-repetition maximum test resultsAfter four weeks of RT, no significant difference was observed in the pups’ body mass (Table 1). Although the groups fed the Western diet had greater body mass before training, there was also no significant difference in the strength of the animals in the WDRT group compared to those in groups C and WDS.
Changes in weight, absolute one-repetition maximum and one-repetition maximum/body mass of animals after the resistance training protocol.
| Period | C (n=7) | WDS (n=7) | WDRT (n=7) | |
|---|---|---|---|---|
| Body mass, g | Initial | 266.3±2.4 | 280.3±6.8 | 277.3±4.0 |
| Final | 300±3.5 | 308.4±5.4 | 309.1±3.7 | |
| 1RM, g | Initial | 1340±58.1 | 1420±81.3 | 1480±55.3 |
| Final | 1560±54.1 | 1710±83.5 | 1770±57.8 | |
| 1RM/body mass | Initial | 4.98±0.2 | 5.0±0.4 | 5.3±0.2 |
| Final | 5.11±0.18 | 5.59±0.36 | 5.81±0.25 |
1RM: one-repetition maximum; C: control group; WDRT: Western diet trained group; WDS: Western diet sedentary group.
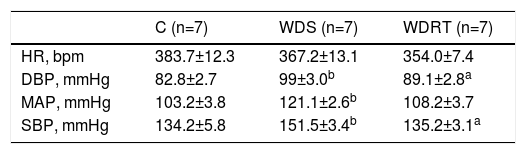
Hemodynamic variables are presented in Table 2. The WDS group had higher BP at rest than groups C and WDRT, while the WDRT group had lower MAP, SBP and DBP than the WDS group. There were no differences in HR between the groups.
Hemodynamic variables recorded 24 hours after the resistance training protocol.
| C (n=7) | WDS (n=7) | WDRT (n=7) | |
|---|---|---|---|
| HR, bpm | 383.7±12.3 | 367.2±13.1 | 354.0±7.4 |
| DBP, mmHg | 82.8±2.7 | 99±3.0b | 89.1±2.8a |
| MAP, mmHg | 103.2±3.8 | 121.1±2.6b | 108.2±3.7 |
| SBP, mmHg | 134.2±5.8 | 151.5±3.4b | 135.2±3.1a |
One-way analysis of variance was used followed by the Bonferroni post hoc test.
C: control group; DBP: diastolic blood pressure; HR: heart rate; MAP: mean arterial pressure; SBP: systolic blood pressure; WDRT: Western diet trained group; WDS: Western diet sedentary group.
The results for autonomic modulation are shown in Figure 1. The WDS group presented an increase in the LF component (Figure 1a) and a reduction in HF (Figure 1b). RT attenuated this alteration, producing a reduction in LF and an increase in HF (Figure 1c).
Cardiovascular autonomic modulation and spontaneous baroreflex sensitivity after four weeks of resistance training. BRS: baroreflex sensitivity; C: control group; HF: high frequency; LF: low frequency; LFSBP: low frequency component of systolic blood pressure; NU: normalized units; WDRT: Western diet trained group; WDS: Western diet sedentary group. ap<0.05 for WDRT vs. WDS; p<0.05 for WDRT vs. C; bp<0.05 for WDS vs. C; cp<0.05 for WDRT vs. C. One-way analysis of variance was used followed by the Bonferroni post hoc test.
In addition, the high LF component of SBP had high values in the WDS group, while for WDRT animals this variable was low (Figure 1d). Although BRS was unchanged as a result of the Western diet, there was an increase in BRS in animals in the WDRT group (Figure 1e) compared to the WDS group.
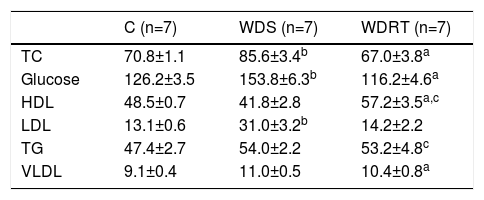
Biochemical variablesThe results for the biochemical variables of fasting blood glucose, TC, HDL, LDL, VLDL and TG can be observed in Table 3. The WDS group had higher fasting blood glucose, TC and LDL than group C, whereas the WDRT group had lower blood glucose, TC and LDL and higher HDL.
Biochemical variables assessed in blood samples collected 24 hours after the resistance training protocol.
| C (n=7) | WDS (n=7) | WDRT (n=7) | |
|---|---|---|---|
| TC | 70.8±1.1 | 85.6±3.4b | 67.0±3.8a |
| Glucose | 126.2±3.5 | 153.8±6.3b | 116.2±4.6a |
| HDL | 48.5±0.7 | 41.8±2.8 | 57.2±3.5a,c |
| LDL | 13.1±0.6 | 31.0±3.2b | 14.2±2.2 |
| TG | 47.4±2.7 | 54.0±2.2 | 53.2±4.8c |
| VLDL | 9.1±0.4 | 11.0±0.5 | 10.4±0.8a |
One-way analysis of variance was used followed by the Bonferroni post hoc test.
C: control group; HDL: high-density lipoprotein; LDL: low-density lipoprotein; TC: total cholesterol; TG: triglycerides; VLDL: very low-density lipoprotein; WDRT: Western diet trained group; WDS: Western diet sedentary group.
The results of the present study show that a Western diet fed to Wistar rats during the perinatal period had important physiological effects, including increased serum glucose, TC, and LDL and reduced HDL. The Western diet also led to dysautonomia, promoting cardiovascular sympathetic modulation and hypertension in adulthood. These findings corroborate previous results from our laboratory using the same experimental protocol and diet.9
Low-intensity RT (40% of 1RM) does not lead to alterations in body mass or strength gain, although individuals on fitness programs show changes in body composition.17,18 The American College of Sports Medicine (ACSM)14 recommend, in RT programs aimed at increasing muscle strength, loads corresponding to 60-70% of 1RM for novice to intermediate individuals and 80-100% of 1RM for advanced individuals. Programs designed for increasing muscular hypertrophy should use loads of 70-85% of 1RM for novice and intermediate individuals and 70-100% for advanced training. Baraúna et al. observed increased muscle strength in mice trained on apparatus similar to that used in our study, although using workloads of 75% of 1RM, supporting the ACSM's recommendations.14 Our results show that the intensity and duration of RT used in the present study did not lead to modifications in body mass or strength in these animals.
To our knowledge, this is the first study to show that low-intensity RT reduces sympathetic modulation, increases BRS and improves the lipid and glycemic profiles of animals exposed to a Western diet in the perinatal period, which did not develop the hypertension observed in sedentary animals on the same diet. Animals exposed to a Western diet and to RT presented lower BP and improvements in most biochemical variables. RT improved BRS, without increasing the LF component but increasing the HF component of PI. The mechanisms behind this effect may be associated with adjustments in central BP control mechanisms.
Physical exercise has been associated with various cardiovascular adaptations, including reduced vascular resistance,19,20 cardiac remodeling, lower blood pressure, reduced HR21 and increased expression of endothelium-derived relaxing factors.22
It has been shown that aerobic exercise induces neural changes in autonomic nuclei, increasing noradrenergic signaling from the nucleus of the solitary tract (NST) to pre-autonomic neurons in the paraventricular nucleus (PVN) of the hypothalamus, increasing the intrinsic excitability of neurons in the PVN-NST pathway, and increasing vagal tone through the release of acetylcholine by the vagus nerve.23,24 The same training model leads to an increase in mRNA of the oxytocin receptor in the medulla and greater expression of this neurotransmitter in the PVN,23,25,26 which correlates with improvement in baroreflex control.24,27 These adjustments may help to reduce sympathetic tone in hypertensive subjects.23
We observed that RT also led to beneficial adaptations in the cardiovascular system, including lower BP, increased BRS and improvement in sympathovagal balance, corroborating previous findings by our group,10 although the mechanisms involved in these responses remain to be elucidated.
Sedentary animals that consumed a Western diet at the start of life presented increased vascular sympathetic modulation, as observed in a previous study carried out in our laboratory,9 while this variable decreased in the group that performed RT. The consumption of high-calorie and high-fat diets is directly related to increases in adipose tissue and obesity, which leads to sympathetic hyperactivity, promoting peripheral vasoconstriction and hypertension.28 There is evidence that reduced sympathetic action in blood vessels helps to decrease BP, mediated by changes in vascular stiffness.29
Previous studies carried out in our laboratory using the same exercise model showed that RT enhances acetylcholine- and insulin-induced vasodilation, increases the bioavailability of nitric oxide, and improves sympathovagal balance and vasodilation capacity. Collectively, these factors help reduce BP.10,30–32
The nutritional insult induced in the animals at the start of life caused metabolic disorders, with increases in TC, LDL and blood glucose, as previously observed.9,33 In models of obesity, insulin resistance is associated with reduced expression of glucose transporter 4 (GLUT4) and impairment of the insulin signaling pathway.34 RT increases the expression of GLUT4, improving insulin response in skeletal muscle and adipose tissue.32,35,36 The reduction in blood glucose observed in this study probably results from improved glucose transportation through GLUT4 translocation.
The trained rats exposed to a Western diet had lower LDL, corroborating studies in which RT reduced this variable in different populations.37,38 Higher serum lipid concentrations increase arterial fat deposits and impair vascular elasticity caused by LDL deposition, which may evolve to atherosclerosis.39
No change was noted in HDL as a result of the consumption of the Western diet. However, the trained animals showed an increase in HDL, which helps to protect the vascular bed. This shows that RT can have an antiatherogenic role.
ConclusionsTo summarize, our results showed that a Western diet at the perinatal stage leads to dysautonomia and metabolic disorders, resulting in hypertension in adulthood, while an RT protocol reduced BP through positive adjustments in autonomic control mechanisms and improved BRS. Furthermore, RT reduced blood glucose and TC and improved the HDL/LDL ratio.
Understanding the mechanisms by which RT improves autonomic control and biochemical profiles in experimental animals offers new possibilities for the prevention and treatment of cardiometabolic disorders caused by inappropriate diet. Further studies should be conducted to elucidate the molecular and epigenetic mechanisms involved in the genesis of metabolic disorders and to understand how exercise can influence central control mechanisms, going beyond endothelial mechanisms based on the release of vasoactive substances.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the Coordination for the Improvement of Higher Education Personnel (CAPES) and the Foundation for Scientific and Technological Research of Sergipe (FAPITEC).
Please cite this article as: Santana MNS, De Melo VU, Macedo FN, et al. Treinamento resistido melhora controle autonômico cardiovascular e perfil bioquímico de ratos expostos a dieta ocidental no período perinatal. Rev Port Cardiol. 2019;38:337–345.


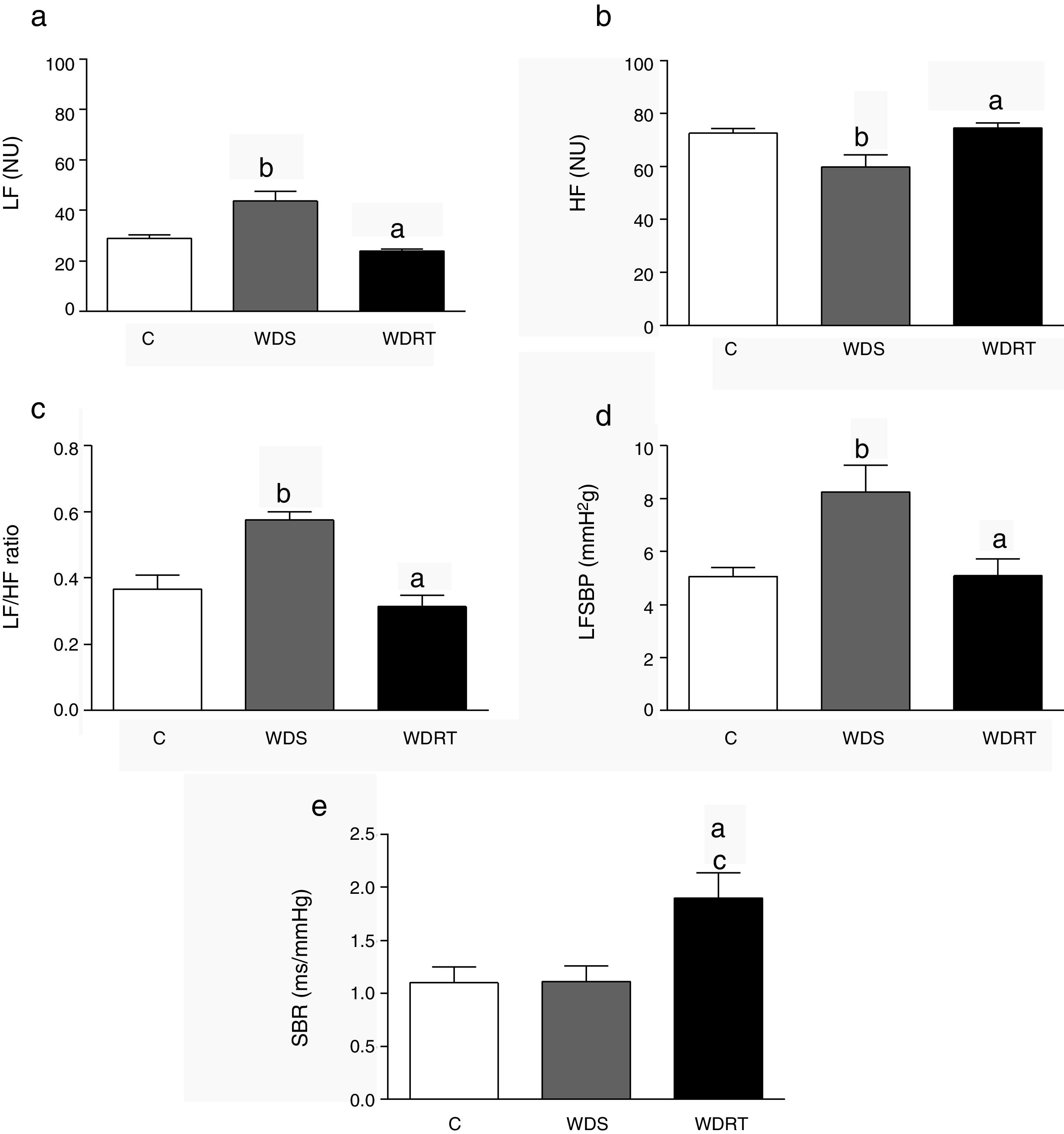
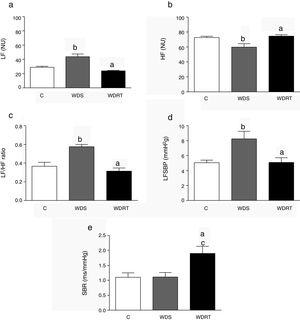 BRS: baroreflex sensitivity; C: control group; HF: high frequency; LF: low frequency; LFSBP: low frequency component of systolic blood pressure; NU: normalized units;
BRS: baroreflex sensitivity; C: control group; HF: high frequency; LF: low frequency; LFSBP: low frequency component of systolic blood pressure; NU: normalized units; 

