The aim of this study was to assess the efficacy and potential complications of a remote-controlled magnetic navigation system (Niobe II, Stereotaxis) for mapping and ablation of right or left ventricular outflow tract ventricular tachycardia or premature ventricular contractions.
MethodsWe studied 32 consecutive patients, mean age 43±11 years, 24 female. Mapping of the arrhythmia was performed using the CARTO RMT mapping system, remotely guided by the Niobe II. Radiofrequency ablation was performed at the site of earliest ventricular activation with pacemapping of at least 11/12 leads.
Acute success was defined as suppression and non-inducibility of the arrhythmia after stimulation with isoprenaline. After a minimum 3-month follow-up, we assessed clinical success (absence of symptoms and suppression of the arrhythmia on Holter recording), defined as less than 50 premature ventricular contractions/24 hours.
ResultsThe origin of the arrhythmia was in the right ventricular outflow tract in 28 patients (88%), in the left in three, and in the epicardium in one. Acute success was achieved in 26 patients (81%). Two patients underwent a second successful procedure, in one of which an epicardial approach was necessary. The overall clinical success rate, after two repeat procedures, was 88%. No complications occurred.
There were two recurrences during a mean follow-up of 307±204 days.
ConclusionThe Niobe II remote control system for mapping and ablation of ventricular outflow tract arrhythmias is effective and safe, and provides precise mapping and a high success rate, with no complications.
Avaliar a eficácia e segurança do sistema de navegação magnética por controlo remoto Niobe II (Stereotaxis) na ablação de taquicardia ventricular ou extrassístoles ventriculares da câmara de saída do ventrículo direito e esquerdo.
MétodosEstudaram-se 32 doentes consecutivos, idade média 43±11 anos, 24 mulheres referenciados para ablação. O mapeamento da arritmia foi efetuado com o sistema CARTO RMT, orientado por controlo remoto. A ablação foi realizada com radiofrequência no local de ativação mais precoce com pacemapping de pelo menos 11/12. O sucesso foi definido como a supressão da arritmia e a sua não inducibilidade sob isoprenalina.
Após um seguimento mínimo de 3 meses avaliámos o sucesso clínico, definido como a ausência de sintomas e a supressão de arritmias no Holter, definido como < 50 extrassístoles ventriculares/24 h.
ResultadosEm 28 doentes, a arritmia originava-se na câmara de saída do ventrículo direito (88%), em 3 no esquerdo, e noutro no epicárdio. O sucesso agudo foi obtido em 26 doentes (81%). Dois doentes efetuaram um segundo procedimento com sucesso, um deles por abordagem epicárdica. O sucesso final, após 2 procedimentos em 2 doentes, foi de 88%. Não ocorreram complicações. Durante um período de seguimento de 307±204 d, ocorreram 2 recorrências.
ConclusõesO sistema de navegação magnética por controlo remoto mostrou-se eficaz e seguro para mapeamento e ablação de arritmias das câmaras de saída ventricular, permitindo um mapeamento preciso com uma elevada taxa de sucesso e sem complicações.
earliest activation site
left ventricular outflow tract
magnetic navigation system
premature ventricular contractions
radiofrequency
right ventricular outflow tract
ventricular tachycardia
Ventricular tachycardia (VT) and idiopathic premature ventricular contractions (PVCs) of the right (RVOT) or left ventricular outflow tract (LVOT) are common, accounting for 10% of all ventricular arrhythmias.1
The focal nature of these arrhythmias makes ablation an effective treatment, with success rates between 81% and 100%.2,3
The standard technique to locate the origin of an arrhythmia is activation mapping during VT or PVCs, together with pacemapping of the earliest activation site (EAS).3 However, manipulation of conventional catheters in the ventricular outflow tract is a lengthy and difficult process due to catheter stiffness, and is not without risk of perforation, which is reported in 1–5% of cases.4,5
Two remote navigation systems have recently been developed aimed at improving catheter manipulation and reducing radiation exposure – the Niobe II magnetic navigation system (MNS) (Stereotaxis)6 and the Hansen robotic remote navigation system (Sensei).7 Clinical experience with the latter for VT ablation is limited,8 and while initial studies on the Niobe II MNS for VT/PVCs have been promising, they were mainly on small series, only 13 and eight patients respectively,9,10 or case reports.11 Other studies have included a larger number of patients but presented no follow-up data.12,13
The aim of this study was to assess the efficacy and potential complications of the MNS for ablation of VT/PVCs originating in the RVOT or LVOT, with a larger number of patients and longer follow-up.
MethodsPatient selectionBetween July 2008 and June 2011, 32 consecutive patients underwent electrophysiological study in our institution, followed by ablation of symptomatic VT or PVCs of probable origin in the RVOT or LVOT.
No patient was contraindicated for magnetic navigation, and all gave their informed consent.
Electrophysiological studyPatients were assessed after six hours’ fasting and without sedation. All antiarrhythmic medication was suspended at least five half-lives before the electrophysiological study; no patient was taking amiodarone.
Surface electrocardiograms and intracavitary electrograms were recorded on an AXIOM Sensis system (Siemens Systems). Programmed stimulation was performed using a UHS 3000 heart stimulator (Biotronik). The catheters were inserted via the femoral vein and positioned under fluoroscopic guidance in the His bundle and in the great cardiac vein via the coronary sinus. Isoprenaline infusion was titrated until induction of VT/PVCs. The protocol included ventricular stimulation in the apex and RVOT with a basic cycle of 600/500/400 ms and S4 until the refractory period was reached and continuous ventricular and atrial stimulation.
Magnetic navigationAll procedures were performed using an AXIOM Artis (Siemens) fluoroscopic imaging system.
The MNS has been previously described6; it basically consists of two computer-controlled magnets positioned on either side of the fluoroscopy table, which create a magnetic field (0.1 T). The position of the magnets is controlled from a console, the Navigant workstation, which orientates the magnetic field according to vectors selected by the operator. The ablation catheter has three magnets at its distal end, which orientate it parallel to the magnetic field. Changes in the orientation of the magnetic field deflect the catheter, which is advanced remotely by a motor at the proximal end of the catheter (Cardiodrive, Stereotaxis). Magnetic field vectors can be stored, enabling subsequent automatic navigation to previous sites.
Mapping and ablationThe MNS is integrated with a CARTO XP RMT (Biosense Webster) electroanatomical mapping system and receives real-time information on the position and orientation of the mapping catheter tip.
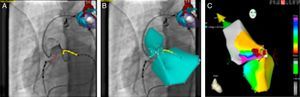
This information is overlaid on the fluoroscopic images on the Navigant workstation, providing real-time monitoring of the catheter position without the need for further fluoroscopy (Figure 1).
Reference fluoroscopic images on the Navigant workstation in right and left anterior oblique views showing the position of the ablation catheter (blue star) in real time without need for further fluoroscopy; MNS automatic vector indicating the right ventricular outflow tract (yellow vector) and the His bundle (yellow dots).
Mapping was performed during VT or in sinus rhythm for those with PVCs. A 4-mm Navistar RMT (Biosense Webster) catheter with three magnets at its tip was used until the Navistar RMT Thermocool (Biosense Webster) magnetic irrigated catheter became available, which was then used in all subsequent procedures. The ablation catheter was introduced via the femoral vein and advanced manually to the right atrium, and then remotely to the His bundle and the RVOT. The locations of the His bundle and the pulmonary valve were marked.
Detailed mapping of ventricular activation was performed in patients in sinus rhythm with PVCs and in those in VT.
Bipolar activation times were reviewed manually and activation maps were generated in automatic mode, the earliest isochrone being considered the EAS. Following isochronal reconstructions of the RVOT and LVOT, pacemapping was performed in multiple endocardial sites close to the EAS. Ventricular stimulation for pacemapping was performed with the same cycle as that of VT or with the coupling interval of the PVCs. Radiofrequency energy (RF) was applied at the EAS showing a QS pattern on the unipolar signal and pacemapping with 11/12 or 12/12 lead match. When mapping of the RVOT did not identify a site meeting these criteria, the LVOT was mapped by introducing the ablation catheter into the left ventricle using a retrograde approach via the femoral artery, with administration of intravenous heparin after arterial puncture.
Using an EP Shuttle RF generator (Stockert), RF was applied between the distal electrode of the ablation catheter and a cutaneous patch electrode for up to 120 s, with a power limit of 55 W and maximum temperature of 55°C in the case of the 4-mm catheter, and power limit of 35 W and maximum temperature of 43°C with the irrigated catheter. When this was ineffective, additional RF applications were performed at sites adjacent to the EAS with good pacemapping. Light sedation with midazolam (bolus) or remifentanil (perfusion) was administered during RF application when necessary.
Procedure and fluoroscopy times were recorded, as well as the number and duration of RF applications. Procedure time was defined as the interval between venous puncture and removal of the introducer.
Success was defined as suppression of PVCs or non-inducibility of VT following stimulation with isoprenaline up to 30 min after ablation.
All patients remained under surveillance in hospital for 24 hours after the procedure.
Follow-upFollow-up consultations included clinical assessment and 24-h Holter monitoring in the first month, at three months and every six months thereafter.
Clinical success was defined as absence of symptoms and suppression of arrhythmias on Holter monitoring (<50 PVCs/24 h).
Statistical analysisData are presented as means ± standard deviation for continuous variables and as frequencies for discrete variables.
ResultsPopulationThirty-two patients, mean age 43±11 years, 24 female, underwent ablation of VT or frequent PVCs.
Six had documented VT, while 26 presented frequent PVCs on 24-h Holter monitoring (minimum of 10 000/24 h) that were symptomatic and refractory to therapy. All patients underwent echocardiographic study, on which none presented left or right ventricular morphological changes. Four patients had hypertension. Sustained VT could not be induced in four of the six patients with documented VT, while well-tolerated VT, with cycles of 388 ms and 400 ms, was induced in two with isoprenaline perfusion.
Mapping and ablationThe RVOT was identified as the origin of the arrhythmia in 28 patients, septal in 20 and in the free wall in eight. The arrhythmia originated in the left ventricle in three patients, one in the right coronary cusp and two in the LVOT. Mapping of the right and left ventricles in one patient failed to identify a sufficiently early activation site, and an epicardial origin was assumed.
Mapping was performed during VT in two patients, and in sinus rhythm in those with PVCs. At the ablation site, the ventricular electrogram preceded the start of the QRS complex by at least 30 ms, with pacemapping of 11/12 or 12/12 leads.
In the three patients with left ventricular arrhythmias, coronary angiography was performed prior to ablation. Remote magnetic navigation was used to map the RVOT and LVOT in all procedures; a 4-mm catheter was used in the first three procedures and an irrigated catheter in the other 31.
During the first seconds of RF application at sites of successful ablation, VT runs of similar morphology to the clinical arrhythmia occurred, and when ablation during VT was performed, the arrhythmia was suppressed during application. Ablation was successful in 26 patients (81%), and was unsuccessful in six; in one patient the EAS was less than 5 mm from the right coronary ostium (Figure 2), and the origin of the arrhythmia was assumed to be epicardial in another.
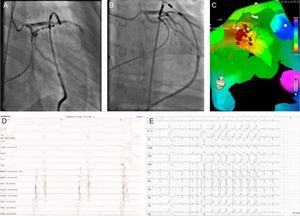
Two patients underwent repeat procedures. A transpericardial approach was used in the patient with an epicardial focus; an irrigated ablation catheter was introduced into the pericardial space through a long sheath and epicardial mapping was performed using the MNS. The EAS was identified in the anterior side of the LVOT next to the anterior interventricular vein. The epicardial electrogram at the ablation site preceded the QRS complex by 42 ms with pacemapping of 12/12 leads; following coronary angiography, RF ablation at this site suppressed the EAS (Figure 3). The other patient, with the EAS in the RVOT, underwent successful repeat ablation 11 months after the first procedure. The overall success rate, after repeat procedures in two patients, was 88%. Mean procedure time was 208±79 min and mean fluoroscopy time was 10±7.8 min. An average of 9±7 applications per patient were performed, with a mean RF application time of 460±290 s.
Epicardial ablation: catheter at the ablation site in right (A) and left (B) anterior oblique views. Activation map (C) showing earliest activation site in the anterior side of the left ventricular outflow tract next to the anterior interventricular vein (red dots). Electrogram and pacemapping with 12/12 lead match at the site of successful ablation (D and E).
No complications related to remote mapping or ablation were observed. During a mean follow-up of 307±204 days, two patients presented recurrent PVCs on Holter monitoring, one of whom was asymptomatic and PVCs decreased from 24 000/24 h before ablation to 5000/24 h after ablation; the other reported symptoms but refused a repeat procedure.
DiscussionConventional ablation catheters have to negotiate two curves to reach the RVOT, the first between the right atrium and the RVOT and the second between the inflow tract and the outflow tract, which makes manipulation difficult and hinders rapid and accurate mapping.
The Niobe II MNS enables the ablation catheter to negotiate various curves within the cardiac chambers, and the tip can be steered in any direction, thus providing ease and precision of mapping unobtainable with conventional catheters.14 With the MNS, the ablation catheter can easily be positioned at any site in the RVOT or LVOT without the need for long sheaths, enhancing catheter stability, as previously demonstrated.15
The options for automatic mapping (AutoMap) on the Navigant workstation, together with the possibility of returning to previously mapped sites using stored magnetic field vectors without fluoroscopic guidance, reduce overall fluoroscopy times. The mean fluoroscopy time with this method of 10±7.8 min is much shorter than in other studies with conventional ablation using three-dimensional mapping systems (45.9±17.9 min)16 or multielectrode mapping catheters (49.7±26.3 min).17 A study comparing conventional techniques and the MNS in ablation of various type of arrhythmia, including VT, showed significantly less fluoroscopy time with the MNS (30±20 vs. 35±25 min, p<0.01).13 A previous study of VT ablation with the MNS reported shorter fluoroscopy time than in our series (7.5±4.3 min), but it did not include LVOT or epicardial ablation9.
Manipulation of catheters in structures like the LVOT and RVOT carries a risk of perforation.4,5 The flexibility of the MNS catheter, together with its soft tip, reduces the force applied to the endocardium compared to conventional catheters,18 which may explain the absence of complications in this and other studies.9–13
LimitationsThe present study was not randomized and included a small number of patients, and no comparison was made with conventional ablation in terms of procedure or fluoroscopy times or efficacy.
ConclusionThe remote control Niobe II system for mapping and ablation of arrhythmias originating in the ventricular outflow tracts was effective and safe in our patient group, with reduced fluoroscopy times and no complications.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Parreira L, et al. Ablação de arritmias da câmara de saída do ventrículo direito e esquerdo com sistema de navegação magnética por controlo remoto. Rev Port Cardiol. 2013. http://dx.doi.org/10.1016/j.repc.2012.12.012.



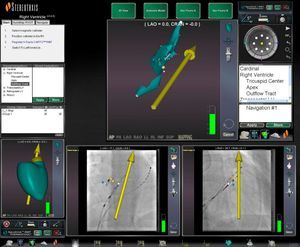 MNS automatic vector indicating the right ventricular outflow tract (yellow vector) and the His bundle (yellow dots).' title='Reference fluoroscopic images on the Navigant workstation in right and left anterior oblique views showing the position of the ablation catheter (blue star) in real time without need for further fluoroscopy;
MNS automatic vector indicating the right ventricular outflow tract (yellow vector) and the His bundle (yellow dots).' title='Reference fluoroscopic images on the Navigant workstation in right and left anterior oblique views showing the position of the ablation catheter (blue star) in real time without need for further fluoroscopy;