Percutaneous closure of the left atrial appendage (LAA) is a promising therapy in patients with atrial fibrillation with high risk for stroke and contraindication for oral anticoagulation (OAC). Intracardiac echocardiography (ICE) may make this percutaneous procedure feasible in patients in whom transesophageal echocardiography (TEE) is inadvisable. Our aim was to assess the efficacy and safety of LAA closure and the feasibility of ICE compared to TEE to guide the procedure.
MethodsIn this cohort study of patients who underwent LAA closure between May 2010 and January 2017, clinical and imaging assessment was performed before and after the procedure.
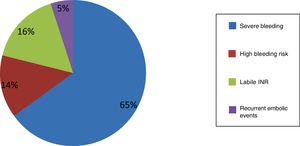
ResultsIn 82 patients (mean age 74±8 years, 64.4% male) the contraindications for OAC were severe bleeding or anemia (65%), high bleeding risk (14%), labile INR (16%), or recurrent embolic events (5%). The procedural success rate was 96.3%. The procedure was guided by TEE or ICE, and no statistically significant differences were observed between the two techniques. During follow-up, one patient had an ischemic stroke at 12 months, two had bleeding complications at six months, and there were four non-cardiovascular deaths. Embolic and bleeding events were less frequent than expected from the observed CHA2DS2VASc (0.6% vs. 6.3%; p<0.001) and HAS-BLED (1.2% vs. 4.1%; p<0.001) risk scores.
ConclusionsIn this population percutaneous LAA closure was shown to be safe and effective given the lower frequency of events than estimated by the CHA2DS2VASc and HAS-BLED scores. The clinical and imaging results of procedures guided by ICE in the left atrium were not inferior to those guided by TEE.
O encerramento percutâneo do apêndice auricular esquerdo (AAE) constitui uma terapêutica de interesse clínico nos doentes de alto risco de acidente vascular cerebral (AVC) e contraindicação para anticoagulação oral (ACO). A ecografia intracardíaca (ICE) pode tornar este procedimento exequível em doentes em que o ecocardiograma transesofágico (ETE) está desaconselhado. Os objetivos consistiram na avaliação da eficácia e segurança da técnica de encerramento do AAE e na avaliação da exequibilidade do ICE em comparação com o ETE para guiar o procedimento.
MétodosEstudo de coorte em doentes submetidos a encerramento do AAE entre maio 2010 e janeiro 2017. Realizada uma avaliação clínica e imagiológica antes e após o procedimento.
Resultados82 doentes (idade 74±8 anos, 63% homens) em que a razão para não realizar ACO foi: hemorragia grave/anemia não controladas (65%), risco hemorrágico elevado (14%), INR lábil (16%) e eventos embólicos de repetição apesar de ACO terapêutica (5%). O procedimento foi guiado por ETE ou ICE. A taxa de sucesso de implantação de dispositivo foi de 96,3%. Foram comparadas as duas técnicas de imagem não se tendo verificado diferenças estatisticamente significativas. No seguimento houve um AVC isquémico, duas complicações hemorrágicas, quatro mortes de causa não cardiovascular. Os eventos embólicos e hemorrágicos foram menos frequentes do que o esperado de acordo com os scores CHA2DS2VASc (0,6% versus 6,3%, p<0,001) e HASBLED (1,2% versus 4,1%, p<0,001).
ConclusõesNesta amostra, o encerramento percutâneo do AAE foi considerado seguro e eficaz comparativamente aos eventos estimados pelo CHA2DS2VASc e HASBLED. Os procedimentos guiados por ICE na aurícula esquerda não tiveram resultados clínicos ou imagiológicos inferiores aos procedimentos conduzidos por ETE.
According to the FAMA study,1 the prevalence of atrial fibrillation (AF) in Portugal is 2.5% in individuals aged ≥40 years. It is more prevalent in the elderly and in those with hypertension, cardiac valve disease, obesity, diabetes and chronic kidney disease.2
Morbidity and mortality resulting from AF are high: it is independently associated with a two-fold increased risk of all-cause mortality and a five-fold increase in risk of stroke.2,3 Embolic stroke is more clinically severe than other causes of brain damage; it is often fatal, and is associated with greater incapacity and recurrence rates.2–8
Although oral anticoagulation (OAC) is effective in preventing stroke, warfarin is contraindicated in 14-44% of patients at risk for cardioembolic stroke,12 while even in eligible patients, only 54% are anticoagulated.9–12 Various factors are responsible for these low figures, the most important of which is bleeding risk, but the need for frequent laboratory monitoring, problems with patient compliance, and concerns among physicians also contribute to the poor performance of this treatment.4
Novel oral anticoagulants (NOACs) have recently been developed: dabigatran, a direct thrombin inhibitor, and the factor Xa inhibitors rivaroxaban, apixaban and edoxaban. Clinical trials (RE-LY for dabigatran,12 ROCKET-AF13 for rivaroxaban, ARISTOTLE14 for apixaban and ENGAGE15 for edoxaban) demonstrated their non-inferiority to warfarin for prevention of thromboembolic events in AF, and the European Society of Cardiology guidelines on the management of AF consider the NOACs to be preferable to warfarin.2 However, these drugs carry significant bleeding risk, which is an obstacle to their use in some patients with more morbidity. Discontinuation rates in the clinical trials (mainly due to intolerance or side effects) were 25.3% for apixaban vs. 27.5% for warfarin in ARISTOTLE,14 34.4% for edoxaban vs. 34.5% for warfarin in ENGAGE,15 21% for dabigatran vs. 17% for warfarin in RE-LY,12 and 23.7% for rivaroxaban vs. 22.2% for warfarin in ROCKET-AF13 (in the latter two trials the discontinuation rate was actually higher for the NOAC than for warfarin). In addition, all these drugs are contraindicated in cases of a history of hemorrhagic stroke, uncontrolled non-intracranial bleeding, and end-stage chronic renal disease or dialysis.
In non-valvular AF most thrombi originate in the left atrial appendage (LAA).2 Percutaneous LAA closure is recommended for patients with non-valvular AF, high stroke risk2 and contraindication for OAC. The feasibility of this procedure has been demonstrated in multiple clinical trials using a variety of closure devices.4,8,9,16–22 Bearing in mind the invasive nature of percutaneous LAA closure, patients for whom it presents a good risk/benefit ratio are those with high thromboembolic risk and contraindication for or failure of anticoagulant therapy.16–22 In previous reports of percutaneous closure, patients required only antiplatelet therapy, rather than anticoagulation, following implantation.18–25
The primary objective of the present study was to assess the efficacy and safety of LAA closure for stroke prevention in patients with non-valvular AF and failure of or contraindication for OAC. The secondary objective was to compare procedures guided by intracardiac echocardiography (ICE) compared to transesophageal echocardiography (TEE).
MethodsPatient selectionBetween May 2010 and January 2017, 82 patients with non-valvular AF (64.6% permanent, 4.9% persistent, 30.5% paroxysmal) with high embolic risk (CHA2DS2VASc score ≥2) and ineligible for OAC due to contraindication or failure of the therapy to prevent thromboembolic events, were selected for percutaneous LAA closure. Written informed consent was obtained from all patients.
Stroke was defined as a focal neurological impairment of sudden onset, and lasting more than 24 hours (or leading to death) and of presumed vascular origin (World Health Organization, 2006).
Bleeding complications were defined and classified according to the Thrombolysis In Myocardial Infarction system.26 Major bleeding was defined as intracranial hemorrhage or blood loss associated with ≥5 g/dl decrease in hemoglobin concentration, minor bleeding as observed blood loss associated with decrease in hemoglobin concentration of ≥3 and <5 g/dl or ≥4 g/dl decrease in hemoglobin concentration with no observed blood loss, and minimal bleeding as any clinically overt sign of hemorrhage associated with a <3 g/dl decrease in hemoglobin concentration.
Chronic kidney disease was defined according to the five stages of the Kidney Disease: Improving Global Outcomes classification.27
Patients were considered to be ineligible for OAC if (1) warfarin or other OAC was contraindicated due to the patient's comorbidities; (2) their international normalized ratio (INR) was labile or impossible to monitor; (3) blood dyscrasia secondary to hematological disease was present; (4) the patient had a history of intracranial bleeding or severe non-intracranial bleeding (not controlled by OAC or antiplatelet therapy); or (5) OAC was shown to be ineffective due to identification of thrombus in the LAA or the occurrence of thromboembolic events despite appropriate OAC.
Protocol for percutaneous left atrial appendage closure and follow-upBefore the procedure, all patients underwent TEE after vascular filling with 500 ml of saline solution to characterize the morphology and to measure the depth and diameters of the LAA and to exclude the presence of thrombus. Implantation was guided by three-dimensional TEE under deep sedation or by ICE under local anesthesia, with the probe located in the left atrium.
In cases guided by TEE, standard views (0°, 45°, 90° and 135°) were used to guide the procedure and to confirm the dimensions of the LAA.
In cases guided by ICE, two femoral accesses were used: left to introduce the ICE catheter, and right to perform LAA closure. The ICE catheter was initially placed in the right atrium and rotated clockwise and tilted back to visualize the fossa ovalis and to guide transseptal puncture inferior and posterior in the fossa. The interatrial septum puncture was then dilated three times with the device delivery sheath to facilitate the passage of the ICE catheter to the left atrium (LA) through a single transseptal puncture. The ICE probe was then advanced with a slight anterior tilt to the LA, over the stiff guidewire in the pulmonary veins. In the LA, the ICE probe was positioned parallel to the LAA, rotated clockwise and tilted posteriorly, and three views were acquired, similar to those used for TEE: mitral view, without angulation of the ICE probe (corresponding to the 80-120° TEE view); aortic view, tilting the probe back (corresponding to the 0-50° TEE view); and posterior view, with the probe tilted sharply and parallel to the left superior pulmonary vein (corresponding to the 110-135° TEE view). ICE was used to guide transseptal puncture, cannulation of the delivery sheath in the LAA, controlled release of the device in the LAA, and assessment of the position of the radiopaque marker bands before final release of the occlusion device.
The two imaging techniques were compared in terms of device implantation success rates, two-dimensional and Doppler visualization, procedure and fluoroscopy time, complications, and length of hospital stay. Two occlusion devices were used: the AMPLATZER Cardiac Plug (ACP) or Amulet (St. Jude Medical, Plymouth, MN, USA); and the WATCHMAN (Boston Scientific, Plymouth, MN, USA). Device size was selected in accordance with TEE and angiographic measurements of the LAA in two views (left 20°/cranial 20°, and left 20°/caudal 20°). In cases guided by ICE, device size was selected on the basis of TEE measurements (on the previous day), angiographic measurements, and those acquired by ICE.
The therapeutic protocol following LAA closure consisted of dual antiplatelet therapy (aspirin 100 mg daily and clopidogrel 75 mg daily) for one month and single antiplatelet therapy for six months. Antithrombotic therapy could thereafter be discontinued at the discretion of the attending physician. All patients underwent transthoracic echocardiography (TTE) on the day after the procedure to exclude pericardial effusion, device dislodgement or migration, and peri-device flow. TEE was repeated one month following the procedure to screen for signs of incomplete endothelialization (peri-device flow ≥3 mm), formation of thrombus on the device, device dislodgement or migration, or signs of compression of the left superior pulmonary vein or the circumflex coronary artery, as indicated by changes in wall motion in the irrigated territory.
Clinical follow-up was maintained for 23±1 months, recording the occurrence of adverse events including death and stroke or transient ischemic attack (TIA), and with repeat TTE at three, six, nine and 12 months post-procedure. In the event of complications detected on the echocardiographic assessment, further control TEE exams could be scheduled at the discretion of the echocardiographer.
Complete clinical and echocardiographic follow-up was achieved and no patients were lost to follow-up, the cohort at the end of follow-up consisting of 82 patients.
Statistical analysisA descriptive analysis was performed of continuous variables, which were calculated as mean and standard deviation, and of categorical variables, which were described as absolute and relative frequencies. Relative frequencies were expressed as percentages rounded to one decimal place. IBM SPSS version 20.0 was used for the statistical analysis.
ResultsCharacteristics of the study populationThe study population consisted of 82 patients with a history of non-valvular AF, of whom 64.6% were male and mean age was 74±8.0 years. The mean CHA2DS2VASc stroke risk score in this population was 4.7±1.4 and the mean HAS-BLED bleeding score was 3.3±1.0 (≥3 in 67.1% of patients). A history of ischemic stroke and severe bleeding was observed in 41.5% and 59.7% of patients, respectively.
The main characteristics of the study population and contraindications for OAC are shown in Table 1 and Figure 1. The most frequent indication for LAA closure was major bleeding under OAC (63.0% under warfarin and the remainder under NOACs). Another important indication was labile INR, which included patients with multiple bleeding episodes without clinical relevance (ecchymosis or hematoma on the limbs) associated with difficulty in monitoring INR or in maintaining it within the therapeutic range (2.0-3.5) (>50% of measurements in consecutive assessments) and those who were unable to have their INR monitored at least once per month, together with the physician's decision not to continue to prescribe warfarin. It should be noted that most cases of labile INR occurred in the early part of the study (2010-2013), when there was less use of NOACs.
Characteristics of the study population.
| Age, years | 74±8.0 |
| Male gender, n (%) | 53 (64.6%) |
| Type of AF, n (%) | |
| Permanent | 53 (64.6%) |
| Persistent | 4 (4.9%) |
| Paroxysmal | 25 (30.5%) |
| CHA2DS2VASc score | 4.7±1.4 |
| HAS-BLED score | 3.3±1.0 |
| HAS-BLED score ≥3, n (%) | 55 (67.1%) |
| History of ischemic stroke, n (%) | 34 (41.5%) |
| History of bleeding events, n (%) | 49 (59.7%) |
| Gastrointestinal | 20 (24.3%) |
| Intracranial | 14 (17.1%) |
| Recurrent epistaxis | 9 (11.0%) |
| Urinary tract | 6 (7.3%) |
| Hypertension, n (%) | 71 (86.6%) |
| Dyslipidemia, n (%) | 35 (42.7%) |
| Type 2 diabetes, n (%) | 26 (31.7%) |
| CKD, n (%) | 23 (28.0%) |
| G3a: GFR 45-59 ml/min/1.73 m2 | 8 (9.7%) |
| G3b: GFR 30-44 ml/min/1.73 m2 | 5 (6.1%) |
| G4: GFR 15-29 ml/min/1.73 m2 | 4 (4.9%) |
| G5: GFR <15 ml/min/1.73 m2 | 6 (7.3%) |
| RRT (hemodialysis) | 8 (12.2%) |
| History of CAD, n (%) | 18 (22.0%) |
| Smoking, n (%) | 11 (13.4%) |
| COPD, n (%) | 8 (9.7%) |
AF: atrial fibrillation; CAD: coronary artery disease; CKD: chronic kidney disease according to the Kidney Disease: Improving Global Outcomes classification; COPD: chronic obstructive pulmonary disease; GFR: glomerular filtration rate; RRT: renal replacement therapy.
Of the 82 patients selected, percutaneous LAA closure was not possible in three (3.6%), two due to the small size of the LAA and in the third due to the presence of severe venous disease; the procedural success rate was thus 96.3%. The mean device size implanted was 23.0±3.0 mm.
Table 2 presents the main technical characteristics of the LAA closure procedure.
Technical characteristics of the left atrial appendage closure procedure.
| LAA | |
| Area by TEE, cm2 | 5.6±1.8 |
| Depth by TEE, mm | 29.8±13.6 |
| Grade ≥3 SEC | 22 (26.8%) |
| Morphology, n (%) | |
| Windsock | 52 (63.4%) |
| Chicken wing | 14 (17.1%) |
| Cactus | 12 (14.3%) |
| Cauliflower | 4 (4.9%) |
| Lobes, n (%) | |
| 1 | 56 (68.3%) |
| ≥2 | 26 (31.7%) |
| Devices | |
| Patients selected, n (%) | 82 (100%) |
| Patients implanted, n (%) | 79 (96.3%) |
| Implantation success, n (%) | 79 (96.3%) |
| Device, n (%) | |
| Amulet | 44 (55.7%) |
| ACP | 32 (40.5%) |
| WATCHMAN | 3 (3.8%) |
| Size of device, mm | |
| Amulet | 24.2±3.6 |
| ACP | 22.6±5.1 |
| WATCHMAN | 26.0±1.7 |
ACP: AMPLATZER Cardiac Plug; LAA: left atrial appendage; SEC: spontaneous echo contrast; TEE: transesophageal echocardiography.
With regard to the imaging modality used during the procedure, 56 implantations (68.2%) were guided by TEE under deep sedation and 26 (31.7%) by ICE under local anesthesia. In recent years (2016-2017) ICE was used more frequently, in 80% of procedures. The imaging modality was not chosen on the basis of the anatomical complexity of the interatrial septum or of the LAA. In our sample, none of the procedures guided by ICE needed to be converted to TEE during the procedure.
The LAA and the implantation procedure were adequately visualized by both methods (Table 3). Procedure and fluoroscopy time were shorter and there were fewer venous access complications in procedures guided by ICE (p<0.001). There were no differences between the methods in closure rates, occurrence of leaks or existence of residual interatrial communication at six months. When the last 10 TEE-guided procedures were compared to the last 10 guided by ICE, statistically non-significant trends were seen in closure success rates (90% vs. 100%, respectively, p=0.07), occurrence of leaks (30% vs. 10%, p=0.09), existence of residual interatrial communication at six months (70% vs. 50%, p=0.06), and complications, both periprocedural and during clinical follow-up (30% vs. 20%, p=0.06).
Comparison between intracardiac echocardiography and transesophageal echocardiography during the procedure and during follow-up.
| TEE | ICE | p | |
|---|---|---|---|
| Modality used, n (%) | 56 (68.2%) | 26 (31.7%) | - |
| Procedure time, min | 69.9±13.6 | 65.8±15.2 | <0.001 |
| Fluoroscopy time, min | 35.1±16.5 | 30.4±17.0 | <0.001 |
| Implantation success, n (%) | 53 (94.6%) | 26 (100%) | 0.08 |
| Periprocedural complications, n (%) | 9 (16.1%) | 3 (11.5%) | 0.001 |
| Complications during follow-up, n (%) | 5 (8.9%) | 0 (0%) | - |
| Occurrence of leaks, n (%) | 5 (8.9%) | 2 (7.6%) | 0.06 |
| Residual interatrial communication at 6 months, n (%) | 29 (35.4%) | 24 (29.3%) | 0.09 |
ICE: intracardiac echocardiography; TEE transesophageal echocardiography.
In the first 24 hours after the procedure there were two major bleeding events (2.4%) with cardiac tamponade requiring pericardiocentesis, and two cases of upper airway bleeding (2.4%) related to tracheal intubation, resolved by conservative measures. Eight small inguinal hematomas (9.8%) at the venous puncture site and one femoral artery pseudoaneurysm (1.2%) were also recorded, all resolved by minimally invasive measures.
No other major periprocedural complications were recorded, including device embolization, stroke/TIA or death.
Clinical follow-upTable 4 presents the major complications that occurred during clinical follow-up.
Complications during clinical follow-up (mean 23±1.0 months).
| Complication | n (%) | Device | When identified |
|---|---|---|---|
| Thrombus on device | 1 (1.2%) | ACP | 1st month |
| Minor leak (<3 mm) | 7 (8.5%) | ACP/Amulet | 1st month |
| WATCHMAN | |||
| Major bleeding | 2 (2.4%) | ACP | 6 months |
| Ischemic stroke | 1 (1.2%) | WATCHMAN | 12 months |
| Death | 4 (4.9%) | ACP/Amulet | 6 and 12 months |
| WATCHMAN |
ACP: AMPLATZER Cardiac Plug.
It should be noted that the patient with thrombus on the ACP device (with no associated shunt) had dual antiplatelet therapy replaced by OAC and that the thrombus regressed completely after six months, with no associated complications, after which the patient returned to single antiplatelet therapy.
A peri-device leak with color jet <3 mm was detected in seven cases (8.5%) but was not confirmed by TEE at three months. There were thus no persistent leaks (>1 month).
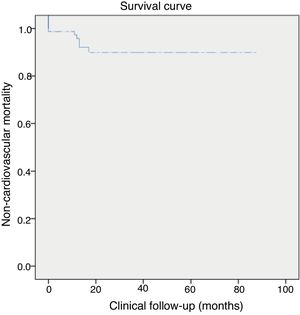
During clinical follow-up (23±1 months, minimum one month and maximum 79 months), there was one ischemic stroke and two major gastrointestinal bleeding events requiring transfusion. Four (4.9%) non-cardiovascular deaths were recorded, three from colon cancer and one from septic shock unrelated to endocarditis 10 months after LAA closure. Clinical follow-up was ≥1 year in 55% of patients. Figure 2 shows all-cause death in the population throughout the study period.
Table 5 presents a comparison between antithrombotic therapy at baseline and one month after LAA closure.
Comparison of antithrombotic therapy at baseline and one month after left atrial appendage closure.
| Drug | Baseline | 1 month |
|---|---|---|
| Aspirin, n (%) | 22 (26.8%) | 63 (76.8%) |
| Clopidogrel, n (%) | 9 (11.0%) | 63 (76.8%) |
| Dual antiplatelets, n (%) | 5 (6.1%) | 63 (76.8%) |
| Warfarin, n (%) | 21 (25.6%) | 3 (3.7%) |
| NOACs, n (%) | 15 (18.3%) | 5 (6.1%) |
| Triple therapy, n (%) | 1 (1.2%) | 1 (1.2%) |
| No therapy, n (%) | 9 (11.0%) | 12 (14.6%) |
NOACs: novel oral anticoagulants.
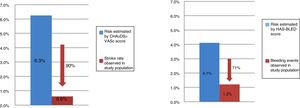
In this population, the mean CHA2DS2VASc stroke risk score was 4.7±1.4, while the mean HAS-BLED bleeding risk score was 3.3±1.0. Embolic and bleeding events were less frequent than expected from the observed CHA2DS2VASc (0.6% vs. 6.3%; p<0.001) and HAS-BLED (1.2% vs. 4.1%. p<0.001) scores (Figures 3 and 4).
DiscussionThis study confirms the feasibility and safety of percutaneous LAA closure in a population of patients with non-valvular AF and high cardioembolic and bleeding risk, in agreement with the literature (Table 6). The three cases of implantation failure in our series occurred at the beginning of our experience with this technique and were related to the small size of the LAA in these patients. With regard to safety, there were two cases (2.4%) of cardiac tamponade requiring pericardiocentesis. The rate of venous access bleeding complications (11.0%) is indicative of the frailty of these patients. Similarly, the overall mortality rate (11.0%) is explained by the advanced age and severe comorbidities seen in this cohort.
Main results of the principal published series using different left atrial appendage closure devices compared with our results.
| Study | Ostermayer et al.19 | Park et al.20 | Holmes et al.(PROTECT AF)8 | Reddy et al.(CAP)23 | Holmes et al.(PREVAIL)24 | Reis et al. (this study) |
|---|---|---|---|---|---|---|
| Device | PLAATO | ACP | WATCHMAN | WATCHMAN | WATCHMAN | ACP and WATCHMAN |
| Patients selected | 111 | 143 | 449 | 460 | 269 | 82 |
| Devices implanted | 108 | 137 | 408 | 437 | 256 | 79 |
| Procedural success | 97.3% | 96% | 90.9% | 95.0% | 95.1% | 96.3% |
| Pericardial effusion requiring peri-cardiocentesis | 5 (4.5%) | 5 (3.6%) | 22 (4.9%) | 10 (2.2%) | 4 (1.5%) | 2 (2.4%) |
| Device embolization | 0 (0%) | 2 (1.4%) | 3 (0.7%) | 0.2% | 2 (0.7%) | 0 (0%) |
| Device thrombus | 1 (0.9%) | NS | 20 (4.9%) | NS | NS | 1 (1.2%) |
| Stroke/TIA | 2 (1.8%)/3 (2.7%) | 3 (2.2%)/0 (0%) | 15 (2.2%)/NS | 0(0%)/NS | 2 (0.7%) | 1 (1.2%) |
| Procedure-related death | 0 (0%) | NS | 2 (0.4%) | NS | NS | 0 (0% |
ACP: AMPLATZER Cardiac Plug; NS: not specified; TIA: transient ischemic attack.
There is no agreement concerning the best antithrombotic protocol in cases of contraindication for or failure of OAC, since such patients were excluded from the only two randomized trials on percutaneous LAA closure (PROTECT AF8,23 and PREVAIL24). LAA closure in patients with contraindication for OAC was analyzed in the ASAP study,28 which showed that the procedure's safety was comparable to previous trials and, in terms of efficacy, there was a reduction of 77% in the occurrence of ischemic stroke compared to that predicted by the CHADS2 score. More recent series29–32 have reported similar results (Table 7). These studies have established percutaneous LAA closure as a useful treatment for patients with high cardioembolic risk who are ineligible for OAC.2
Main thromboembolic and bleeding events reported following left atrial appendage closure with the AMPLATZER Cardiac Plug device in recent studies compared with our results.
| Study | Tzikas et al.29 | Lopez Minguez et al.30 | Urena et al.31 | Jalal et al.32 | Reis et al. (this study) |
|---|---|---|---|---|---|
| Device | ACP | ACP | ACP | ACP | ACP and WATCHMAN |
| No. of patients | 1047 | 167 | 52 | 73 | 82 |
| Antithrombotic therapy | DAPT 1-3 months; aspirin 3 months | DAPT 3-6 months; aspirin 6-12 months | DAPT or SAPT 1-6 months, then SAPT | SAPT | DAPT 1 month; aspirin indefinitely |
| Clinical follow-up, months | 13 [6-25] | 22±8.3 | 20±5.0 | 13±3.0 | 21±1.0 |
| Actual vs. predicted thromboembolic events, %/year | 2.3 vs. 5.6 | 2.4 vs. 8.3 | 3.4 vs. 10.0 | 4.0 vs. 9.9 | 0.6 vs. 6.3 |
| Actual vs. predicted bleeding events, %/year | 2.0 vs. 5.3 | 3.1 vs. 6.6 | 3.4 vs. 8.7 | 1.3 vs. 4.3 | 1.2 vs. 3.3 |
| Device thrombus (%) | 4.4 | 8.0 | 0 | 6.8 | 1.2 |
ACP: AMPLATZER Cardiac Plug; DAPT: dual antiplatelet therapy; SAPT: single antiplatelet therapy.
The present study is the largest published Portuguese series of patients with non-valvular AF undergoing percutaneous LAA closure with a mean follow-up of more than two years. It included patients with more severe thromboembolic and bleeding risk than those in the main published series, as shown by higher mean CHA2DS2VASc and HAS-BLED scores. However, there were fewer embolic and bleeding events in the study sample than expected according to these scores.8,19,31
Antithrombotic therapy is crucial to prevention of stroke and systemic embolism in AF. Its implementation is based on the presence or absence of risk factors. The NOACs are preferred to warfarin for the prevention of embolic events, due mainly to their lower rates of fatal bleeding and hemorrhagic stroke.2 However, these drugs have certain limitations and the discontinuation rate is high.12–14 There is therefore a patient group in need of an appropriate clinical solution.
All OAC is contraindicated in patients with a history of hemorrhagic stroke. Renal excretion of NOACs ranges from 25% for apixaban to 80% for dabigatran, although data are scarce for patients with severe kidney disease, since the main randomized trials on these drugs did not assess their efficacy and safety in patients with glomerular filtration rate <15 ml/mm, and they are thus not recommended for such patients. Renal function worsens with age and therefore renal clearance of oral anticoagulants also decreases. At the same time, increasing age also brings a heightened risk of bleeding.32 As demographic changes lead to aging populations with more combustible and as more patients are identified with non-valvular AF in whom OAC is contraindicated or ineffective, the advantages of percutaneous LAA closure in reducing risk of stroke and severe bleeding become clearer.
This paper reports the first consecutive series of patients undergoing LAA closure in whom ICE was used in the left atrium as a safe and effective method for guiding the procedure. Percutaneous LAA closure is usually monitored by echocardiography, most often by TEE due to its excellent spatial resolution. However, TEE has some limitations, including the need for deep sedation and the risk of complications associated with airway manipulation, which are especially important in patients with more comorbidities. ICE is a particularly valuable imaging technique for patients with severe chronic obstructive pulmonary disease, esophageal varices or gastroesophageal conditions that carry a high risk of bleeding (Table 8).
Advantages and disadvantages of intracardiac echocardiography.
| Advantages of ICE |
| High spatial resolution and safe when TEE is contraindicated |
| Local anesthesia with no need for deep anesthesia or sedation |
| Better workflow in the catheterization laboratory and thus faster patient turnover |
| Fewer complications than with TEE and thus greater patient comfort |
| No need for contrast administrative and thus no cases of contrast nephropathy |
| Faster patient recovery and thus lower hospitalization costs |
| Disadvantages of ICE |
| Additional venous puncture and hence added risk of complications |
| Steep learning curve (easier if the operator already has experience with TEE) |
| Single-use catheter (although it can be reused) |
| Single-plane views only (multiplane and 110-135° 3D views possible with TEE). |
3D: three-dimensional; ICE: intracardiac echocardiography; TEE: transesophageal echocardiography.
Proficiency in both imaging techniques for guiding percutaneous procedures is advantageous in cases in which TEE is unsuitable (Table 9). The choice between ICE and TEE should be based on the patient's anatomy and comorbidities and the institution's anesthesia facilities. Although in our study it was not necessary to change the imaging modality from ICE to TEE during any of the procedures, in cases of complex cardiac anatomy TEE may be the preferred method due to its ability to provide standard echocardiographic views as well as multiplane and three-dimensional images.
Potential contraindications for transesophageal echocardiography.
| Gastroesophageal disorders (tumor, diverticula, scleroderma, symptomatic hiatus hernia) |
| Active or recent upper digestive tract bleeding |
| Esophagectomy |
| Severe cervical arthritis |
| Esophagitis or peptic ulcer |
| Chronic dysphagia |
| Thoracic or abdominal aortic aneurysm |
In our study, ICE was used as a safe and effective method to guide the main steps in percutaneous LAA closure. Device size was selected on the basis of echocardiographic (TEE on the previous day and those acquired by ICE) and angiographic measurements. Visualization of the largest LAA diameter, the landing zone, by ICE cannot be guaranteed in all cases, and it is thus essential to complement LAA measurements with those from other modalities (TEE, cardiac computed tomography and angiography).
Comparison of the two echocardiographic techniques in this sample revealed no significant differences in implantation success or in peri-device leaks on TEE assessment at one month. Procedure and fluoroscopy times and complication rates, including venous access complications, were no higher in the ICE group. In our experience the use of ICE simplified the procedure and reduced the personnel and time required in the catheterization laboratory, as well as decreasing recovery time.
LimitationsThis study has some limitations. It was based on a single center, with a highly selected population at very high cardioembolic and bleeding risk. The number of patients undergoing LAA closure guided by ICE was small, and they were treated at a stage when the technology had matured. Our results do not permit a proper comparison between TEE and ICE in this setting, since the study was not randomized and could therefore have been affected by bias introduced by patient selection and the different stages of maturity of the technique. Nevertheless, even when comparing the last 10 cases guided by each of the two modalities, the results using ICE were not inferior to those of TEE.
Another limitation of the study was that cardiac computed tomography angiography was not performed in these patients, as characterization of the LAA was performed in all cases by TEE.
ConclusionsIn this sample, percutaneous LAA closure was shown to be safe and effective, given the lower frequency of events than estimated by the CHA2DS2VASc and HAS-BLED scores.
The clinical and imaging results of left atrial procedures guided by ICE were not inferior to those guided by TEE.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Reis L, Paiva L, Costa M, et al. Registo de encerramento percutâneo do apêndice auricular esquerdo e experiência inicial com ecografia intracardíaca. Rev Port Cardiol. 2018;37:763–772.