Aortic dilatation can develop late after tetralogy of Fallot repair. Its extension beyond the aortic root is not clearly understood. We aimed to assess the prevalence and predictors of ascending aorta dilatation to set up an imaging protocol.
MethodsIn this prospective study including adult patients after tetralogy of Fallot repair followed at a referral center, we assessed the aorta by cardiovascular magnetic resonance and defined ascending aorta dilatation as an observed-to-expected ratio >1.5.
ResultsWe included 78 adults (mean age 31±10 years; 56% female), with a mean follow-up of 23±7 years since tetralogy of Fallot repair. The prevalence of ascending aorta dilatation was 11.5%. The ascending aorta was larger than the sinuses of Valsalva in 12.8% of cases. Patients with ascending aorta dilatation were older, predominantly male, with later repair and larger left ventricular mass and volumes. By multivariate analysis left ventricular mass index (LVMI) was the only factor independently associated with ascending aorta dilatation (odds ratio 1.10, 95% confidence interval 1.01-1.20, p=0.03). A cut-off value of ≥57.9 g/m2 for LVMI had 89% sensitivity and 71% specificity for ascending aorta dilatation.
ConclusionsAscending aorta assessment as part of a routine cardiovascular magnetic resonance study after tetralogy of Fallot repair is recommended to screen for future aortic complications, particularly in males and older patients, and those with later repair and larger left ventricles. LVMI assessment has potential as a screening tool for ascending aorta dilatation with future clinical implications.
A dilatação da aorta é uma complicação tardia após correção da tetralogia de Fallot. A sua extensão além da raiz da aorta não está bem definida. Pretendemos avaliar a prevalência e os preditores de dilatação da aorta ascendente para elaborar um protocolo imagiológico.
MétodosEstudo prospetivo com adultos operados a tetralogia de Fallot seguidos num centro de referência. Estudamos a aorta por ressonância magnética cardiovascular e definimos dilatação da aorta ascendente pelo rácio observado-esperado > 1,5.
ResultadosIncluímos 78 adultos (idade média 31 ± 10 anos; 56% mulheres); seguimento médio de 23 ± 7 anos desde a cirurgia. A prevalência de dilatação da aorta ascendente foi 11,5%. A aorta ascendente era maior do que os seios de Valsalva em 12,8% dos casos. Os doentes com dilatação da aorta ascendente eram mais velhos, maioritariamente homens, operados mais tarde, com massa e volumes ventriculares esquerdos maiores. Na análise multivariada a massa ventricular esquerda indexada foi a única variável independente associada a dilatação da aorta ascendente (odds ratio 1,10; intervalo de confiança de 95% 1,01-1,20; p = 0,03). A massa ventricular esquerda indexada ≥ 57,9 g/m2 apresentou uma sensibilidade de 89% e uma especificidade de 71% para dilatação da aorta ascendente.
ConclusõesRecomendamos a inclusão da aorta ascendente na avaliação por ressonância magnética da tetralogia de Fallot operada, para prevenir complicações aórticas futuras, em particular em homens, doentes mais velhos, operados mais tarde e com ventrículos esquerdos maiores. A massa ventricular esquerda indexada tem potencial para ser usada no rastreio da dilatação da aorta ascendente com implicações clínicas futuras.
Imaging follow-up of tetralogy of Fallot (TOF) patients can be challenging, due to associated thoracic deformities and previous cardiac surgeries. Cardiovascular magnetic resonance (CMR) has an important role in identifying right ventricular outflow tract obstruction, aneurysms, or residual shunts, quantifying pulmonary valve regurgitation or stenosis, and assessing biventricular systolic function. In addition, as first reported by Capelli et al.,1 there is an increasing awareness that aortic dilatation can develop late after TOF repair. Intrinsic histological abnormalities in the aortic root and ascending aortic wall, present since infancy, can contribute to progressive dilatation.2 This possible aortopathy led us to focus our study beyond the aortic root and reinforces the importance of a complete aortic assessment. The accuracy of CMR in the diagnosis of thoracic aortic disease,3 including TOF,4 is unquestionable. Although CMR is expensive, requires long scan duration and has contraindications, it can provide a complete anatomical and functional assessment.5 We sought to assess the ascending aorta (AAo) late after TOF repair and to find possible predictors of AAo dilatation in order to set up an imaging protocol.
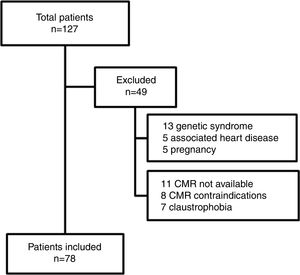
MethodsA total of 127 adults after TOF repair, not including those with pulmonary atresia, are currently followed as regular outpatients at our tertiary care center. This study prospectively included 78 adults after TOF repair, from March 2011 to December 2015. Inclusion criteria were age ≥18 years, with a time interval since TOF repair of>1 year, and ability to undergo a CMR study. Forty-nine patients were excluded from the study (Figure 1). Exclusion criteria were association with other congenital or acquired heart disease, genetic syndromes, pregnancy, and contraindications for CMR. The aorta was assessed by CMR and an observed-to-expected ratio was calculated based on nomograms from Davis et al.6 The population was divided into two groups: group 1, without AAo dilatation (observed-to-expected ratio ≤1.5); and group 2, with AAo dilatation (observed-to-expected ratio >1.5). CMR measurements were indexed to body surface area according to the Du Bois formula.7
Arterial blood pressure was measured twice in supine position at 2-min intervals using an automated blood pressure monitor (SureSigns VS2, Philips Medical Systems) and averaged. It was decided to measure blood pressure in the left brachial artery, due to the presence of a previous right Blalock-Taussig shunt in a significant number (30) of cases. If there was a left palliative shunt, blood pressure was measured on the right arm.
The ratio of stroke volume index to pulse pressure was used as an indirect measure of total systemic arterial compliance.8
Cardiovascular magnetic resonanceCMR imaging was performed using a 3-T system (Siemens Magnetom Trio, Washington DC, USA). CMR image acquisitions and analysis were performed by two experienced investigators blinded to patient data. Electrocardiogram-triggered balanced steady-state free precession cine images were acquired throughout the cardiac cycle in breath-hold. Pulse sequences for a standard ventricular function examination were obtained with the following parameters: field of view 320 mm2; matrix 153×208; voxel size 2.1×1.5×6.0 mm; repetition time 52.9 ms; echo time 1.4 ms; flip angle 60°; slice thickness 6 mm; no gap; temporal resolution 41 ms. Ventricular volumes were assessed using short-axis cine imaging at end-diastole and end-systole applying Simpson's method. The right ventricular outflow tract was included in right ventricular volumes. For left ventricular (LV) mass calculation a combination of semi-automated and manual correction of contours of the endocardial and epicardial borders was used, excluding the papillary muscles. Cine acquisitions aligned with the LV outflow tract in oblique sagittal and coronal orientations were also obtained. In the LV outflow tract sagittal and coronal planes, the maximum end-diastolic external diameter of the aortic segment of interest was measured perpendicular to the longitudinal axis of the aorta, in accordance with the 2010 ACC/AHA guidelines for thoracic aortic disease.3 Contrast angiography and late gadolinium enhancement detection were also performed for both left and right ventricles.
EthicsThe study protocol complies with the Declaration of Helsinki and was approved by the local institutional ethics committee. Written informed consent was obtained from all patients.
Statistical analysisThe statistical analysis was performed using IBM SPSS, version 24.0 (IBM SPSS Inc., Chicago, Illinois, USA). A two-tailed p value <0.05 was considered statistically significant. Continuous data are expressed as mean±standard deviation or as median (range), according to the normality of the variable's distribution. Categorical variables are summarized as frequency and percentage. The normality of the data was verified using histograms and the Kolmogorov-Smirnov or Shapiro-Wilk test as appropriate. Differences between groups were compared with Fisher's exact test and the unpaired Student's t test for categorical and continuous variables, respectively. Intra- and interobserver variability were assessed in 18 randomly selected cases. Bland-Altman analysis was used to determine bias (mean of the difference) with 95% limits of agreement. Pearson's correlation coefficient was estimated to analyze possible associations between AAo dilatation and a number of continuous variables. Receiver operating characteristic curve analysis was used to compute the discriminatory power of AAo dilatation predictors. Multivariate logistic regression was performed to test independent associations with AAo dilatation. Variables associated with probability values <0.1 in univariate analysis were included in the multivariate model.
ResultsThe demographic and hemodynamic characteristics of the study population are listed in Table 1 and CMR data in Table 2, according to the presence or absence of AAo dilatation. Seventy-eight patients (56% female) were included, with a mean age of 31±10 years. The mean follow-up since TOF repair was 23±7 years. Forty-nine patients were excluded (Figure 1). Of note, 15 patients were excluded due to CMR contraindications or claustrophobia.
Demographic and hemodynamic characteristics of the study population according to the presence or absence of ascending aorta dilatation.
| Group 1: no AAo dilatation (n=69) | Group 2: AAo dilatation (n=9) | p | |
|---|---|---|---|
| Age, years | 29.8±8.8 | 39.7±12.9 | <0.01 |
| Male gender, n (%) | 26 (37.7) | 8 (88.9) | <0.01 |
| Right aortic arch, n (%) | 21 (30.4) | 1 (11.1) | 0.23 |
| Previous palliative shunt, n (%) | 35 (50.7) | 4 (44.4) | 1.0 |
| Transannular patch, n (%) | 35 (50.7) | 3 (33.3) | 0.48 |
| Time shunt to repair, years | 3 (1-22) | 3 (2-11) | 0.88 |
| Age at TOF repair, years | 5 (2-49) | 7 (4-21) | 0.02 |
| Follow-up since TOF repair, years | 22.9±6.7 | 24.7±8.5 | 0.48 |
| Systemic arterial hemodynamics | |||
| Systolic blood pressure, mmHg | 115.8±12.2 | 117.7±11.5 | 0.65 |
| Pulse pressure, mmHg | 46.6±7.6 | 47.4±9.4 | 0.77 |
| SAC, ml/mmHg/m2 | 1.0±0.2 | 1.1±0.2 | 0.18 |
Data are expressed as mean ± standard deviation or median (range). Categorical variables are summarized as frequency and percentage. Aortic diameters are measured in mm. AAo: ascending aorta; SAC: systemic arterial compliance; TOF: tetralogy of Fallot.
Cardiovascular magnetic resonance data of the study population according to the presence or absence of AAo dilatation.
| Group 1: no AAo dilatation (n=69) | Group 2: AAo dilatation (n=9) | p | |
|---|---|---|---|
| Aortic measurements | |||
| SoV diameter, mm | 33.6±4.6 | 43.1±6.5 | <0.01 |
| SoV index, mm/m2 | 20.1±2.6 | 23.1±1.9 | <0.01 |
| STJ diameter, mm | 27.7±3.8 | 38.3±5.3 | <0.01 |
| STJ index, mm/m2 | 16.6±2.3 | 20.7±2.0 | <0.01 |
| AAo diameter, mm | 28.6±4.0 | 43.2±5.5 | <0.01 |
| AAo index, mm/m2 | 17.1±2.2 | 23.4±3.4 | <0.01 |
| AAo observed-to-expected ratio | 0.3±0.5 | 2.2±0.7 | <0.01 |
| AAo/SoV ratio >1, n (%) | 5 (7.2) | 5 (55.6) | <0.01 |
| AAo/pulmonary trunk ratio | 1.2±0.3 | 1.5±0.5 | 0.10 |
| Left ventricular study | |||
| LV mass index, g/m2 | 53.3±11.2 | 71.9±12.8 | <0.01 |
| LV mass/volume ratio, g/ml | 0.7±0.1 | 0.8±0.1 | 0.05 |
| LVEDV index, ml/m2 | 76.8±14.8 | 92.1±15.9 | <0.01 |
| LVESV index, ml/m2 | 32.2±9.8 | 41.0±12.4 | 0.02 |
| LV stroke volume index, ml/m2 | 44.6±8.1 | 51.1±10.0 | 0.03 |
| LV ejection fraction, % | 58.5±6.7 | 56.1±8.9 | 0.10 |
| Aortic regurgitation grade III or IV, n (%) | 3 (4.3) | 1 (11.1) | 0.24 |
| Right ventricular study | |||
| RVEDV index, ml/m2 | 129.5±41.5 | 152.9±44.1 | 0.12 |
| RVESV index, ml/m2 | 47.4±19.7 | 52.4±23.2 | 0.48 |
| RV stroke volume index, ml/m2 | 61.0±17.0 | 63.0±10.4 | 0.74 |
| RV ejection fraction, % | 47.9±5.9 | 42.8±7.8 | 0.02 |
| RVEDV/LVEDV ratio | 1.7±0.6 | 1.7±0.5 | 0.81 |
| Pulmonary regurgitation fraction, % | 31.4±17.2 | 39.4±17.4 | 0.22 |
Data are expressed as mean ± standard deviation or median (range). Categorical variables are summarized as frequency and percentage. Aortic diameters are measured in mm.
AAo: ascending aorta; LV: left ventricular; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; RV: right ventricular; RVEDV: right ventricular end-diastolic volume; RVESV: right ventricular end-systolic volume; SoV: sinuses of Valsalva; STJ: sinotubular junction; TOF: tetralogy of Fallot.
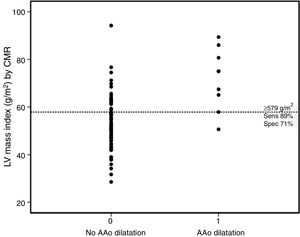
The majority (90%) of patients were asymptomatic, seven were in NYHA functional class II and only one was in class III. A right aortic arch was present in 22 patients. In 39 (50%) a systemic-to-pulmonary shunt was performed prior to complete repair, with a median interval of three years. Thirty-eight patients underwent TOF repair with a transannular patch. Surgical repair was performed in adulthood (age ≥18 years) in nine patients (five male and four female). Five patients had systemic hypertension (three in group 2) but systolic blood pressure and pulse pressure were within normal ranges during the study protocol and follow-up. Twenty-five patients were on medication: six on angiotensin-converting enzyme inhibitors, 10 on beta-blockers, and nine on antiarrhythmics (four on amiodarone, two on digoxin, two on propafenone and one on sotalol). LV stroke volume index and LV ejection fraction were within normal ranges. The prevalence of AAo dilatation was 11.5%, with a maximum absolute AAo diameter ≥40 mm in six cases and ≥50 mm in two, but none above 55 mm. The AAo was larger than the sinuses of Valsalva (SoV) in 12.8%. Patients with AAo dilatation were older, predominantly male, with later TOF repair and larger left ventricular mass and volumes. By multivariate analysis (Table 3) LV mass index (LVMI) was the only independent factor associated with AAo dilatation (odds ratio 1.10, 95% confidence interval [CI] 1.01-1.20, p=0.03). A cut-off value of ≥57.9 g/m2 for LVMI had 89% sensitivity and 71% specificity for AAo dilatation (Figure 2).
Variables associated with ascending aorta dilatation by univariate and multivariate logistic regression analysis.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR | p | OR | p | |
| Age (per year) | 1.10 | <0.01 | 1.07 | 0.09 |
| Male gender | 13.23 | 0.02 | 3.54 | 0.31 |
| Right aortic arch | 0.29 | 0.25 | ||
| Previous palliative shunt | 0.78 | 0.72 | ||
| Transannular patch | 0.55 | 0.44 | ||
| Time from shunt to repair (per year) | 1.05 | 0.67 | ||
| Age at TOF repair (per year) | 1.03 | 0.34 | ||
| Time since TOF repair (per year) | 1.04 | 0.48 | ||
| Systolic arterial pressure | 1.01 | 0.65 | ||
| Diastolic arterial pressure | 1.01 | 0.72 | ||
| Pulse pressure | 1.01 | 0.77 | ||
| SAC | 7.00 | 0.19 | ||
| LV mass index | 1.13 | <0.01 | 1.10 | 0.03 |
| LVESV index | 1.09 | 0.04 | 0.98 | 0.80 |
| RVEDV/LVEDV ratio | 0.86 | 0.81 | ||
AAo: ascending aorta; LV: left ventricular; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; OR: odds ratio; RVEDV: right ventricular end-diastolic volume; SAC: systemic arterial compliance; TOF: tetralogy of Fallot.
Dot diagram for left ventricular mass index according to the presence or absence of AAo dilatation. The horizontal dotted line represents the cut-off point for the best diagnostic accuracy (cut-off ≥57.9 g/m2, with sensitivity of 88.9% and specificity of 71.0%). AAo: ascending aorta; CMR: cardiovascular magnetic resonance; LV: left ventricular; Sens: sensitivity; Spec: specificity.
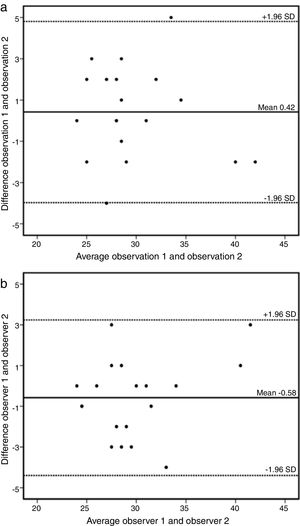
A sample of 18 randomly chosen cases was reanalyzed. Intraobserver variability of the AAo diameter measurement was 0.42 mm (95% CI: -3.97 to 4.81) (Figure 3a) and interobserver variability was -0.58 mm (95% CI: -4.40 to 3.24) (Figure 3b).
(a) Bland-Altman plot of intraobserver variability of ascending aorta data by cardiovascular magnetic resonance. Estimated bias 0.42 mm (95% confidence interval -3.97 to 4.81mm). SD: standard deviation. (b) Bland-Altman plot of interobserver variability of ascending aorta data by cardiovascular magnetic resonance. Estimated bias -0.58 mm (95% confidence interval -4.40 to 3.24mm). SD: standard deviation.
To our knowledge this is the largest prospective CMR study to include only TOF patients without pulmonary atresia. With this exclusion condition we aimed to avoid a potential bias, as in this patient subgroup it is more common to find aortic regurgitation and aortic root dilatation after repair.9 Additionally, we analyzed demographic and anatomical parameters that could be used as predictors of aortic dilation in future research.
A wide range of prevalence of aortic dilatation in TOF has been published. This is probably due to differences in patient selection and definition criteria. Similarly to a previous retrospective study by Kay et al.,10 we defined AAo dilatation as an observed-to-expected ratio >1.5. They found a 6% prevalence of AAo dilatation, but used regression equations developed for computed tomography. In a multicenter study using transthoracic echocardiography,11 the authors reported a 6.6% prevalence of aortic root dilation using the same cut-off ratio for the SoV. In a subgroup of patients the authors had access to the AAo diastolic diameter and reported an estimated prevalence of 18.7% for AAo dilatation, using an absolute diameter cut-off value of ≥40 mm. Although absolute aortic diameters are used for surgical decision,12 adjusting for a gender-specific nomogram derived from a normal CMR reference range appears to be more precise for definition and long-term follow-up in congenital heart diseases, especially in a young population with expected long-term survival. Similarly to previous CMR retrospective studies,10,13 in our cohort eight out of nine patients with AAo dilatation were male. Male gender appears to be the most consistent predisposing factor for AAo dilatation by univariate analysis. However, in our multivariate logistic regression model only LVMI was independently associated with AAo dilatation.
It has been postulated that long-standing volume overload of the aorta before complete TOF repair may be responsible for progressive aortic dilatation. In our cohort, patients with AAo dilatation had higher LV stroke volume index and longer time to TOF repair. Interestingly, nine patients underwent TOF repair in adulthood, due to late diagnosis in four cases (the oldest at age 49 years), and late referral or loss to follow-up during pediatric age in five cases. None showed Ao dilatation at the time of TOF repair. In addition, histopathological abnormalities, mainly in the aortic root and ascending aortic wall, can contribute to aortic dilatation and stiffness in TOF.2,14 Grotenhuis et al.15 and Rutz et al.16 reported aortic dilatation and reduced aortic distensibility as a marker of an intrinsic aortopathy in TOF, compared with normal controls. Alterations in elastic properties were especially found in the proximal aorta,17 and were thought to precede aortic dilation and to increase LV afterload.18,19 Consequently, TOF aortopathy can lead to LV remodeling as a result of ventricular-arterial coupling. Increased LV end-diastolic pressure due to increased LV volumes and afterload could explain why in our study patients with AAo dilatation had a higher LVMI. Moreover, systemic hypertension was more prevalent in the dilated group, although pulse pressure and systemic arterial compliance were similar in the two groups, possibly reflecting good blood pressure control under medication. These findings highlight the need for regular clinical follow-up along with careful imaging follow-up of the aorta and LV parameters, including LV size and function, after TOF repair. We found a larger aortic diameter at the level of the AAo in 13% of patients, demonstrating the importance of complete aortic screening. Although echocardiography is relatively inexpensive, portable and widely available, it has limitations concerning AAo imaging. CMR and computed tomography are the current gold standard for a complete aortic assessment, however CMR imaging has the advantage of avoiding exposure to either radiation or iodinated contrast, compared to computed tomography, in young patients in need of long-term follow-up. The role of CMR for TOF follow-up is unquestionable due to its accuracy and reproducibility. A comprehensive CMR acquisition after TOF repair enables not only screening for residual RV outflow tract and pulmonary valve lesions, but also assessment of right and left ventricular parameters, including LV mass and volumes, and can accurately provide serial aortic diameters. A slow rate of aortic diameter progression in TOF has been reported,10,13 but with a higher rate of progression in the AAo.13 It has been proposed that CMR imaging of the aorta should be repeated every three years if the aorta is not dilated at baseline.10 Although aortic dissection is rare, it can occur long after TOF repair. In the first such case, aortic dissection was reported beginning in the AAo in a 30-year-old man who had undergone TOF repair nine years before.20 To prevent this complication, a complete assessment of the thoracic aorta should be included in a CMR imaging protocol for TOF. Based on our findings, we suggest a careful imaging follow-up of the AAo after TOF repair, especially in males, older patients, and those with later TOF repair and larger left ventricles.
Study limitationsThis was a single-center study and data may have been influenced by selection and survivor bias. We excluded patients with genetic syndromes including DiGeorge syndrome but 22q11 mutations were not screened in all patients. Finally, 15 patients did not undergo CMR due to contraindications or claustrophobia, which represents a limitation of the CMR studies.
Further longitudinal studies with larger population and the use of a consensus definition for aortic dilatation may help to identify patients at risk of aortic events and may clarify the timing for aortic surgery in TOF patients after repair.
ConclusionsAAo assessment as part of a routine CMR study after TOF repair is recommended to prevent future aortic complications, particularly in males, older patients, and those with later repair and larger left ventricles. LVMI assessment has potential as a screening tool for AAo dilatation with future clinical implications.
Conflicts of interestThe authors have no conflicts of interest to declare.
We wish to thank the Radiology Department staff for their dedication and support regarding congenital heart disease patients, who can be sometimes very challenging.