Recurrent right-sided heart failure after pericardiectomy may be caused by incomplete pericardiectomy, recurrent constriction, diastolic dysfunction or myocardial involvement. Identifying recurrent constrictive pericarditis (CP) in patients who have recurring symptoms after pericardiectomy is challenging, since the characteristic Doppler echocardiographic features may not be present if a portion of the ventricles are free of constricting pericardium, and there are no diagnostic or treatment guidelines for management of recurrent CP.
The authors report the case of a 59-year-old man with a history of pericardiectomy for tuberculous CP in 1984, admitted to our hospital with signs and symptoms of right heart failure. After a complete diagnostic workup, recurrent CP was diagnosed. Given the scarcity of cases reported on this disease, three possible therapeutic approaches are discussed: a second pericardiectomy, heart transplantation and medical therapy.
Insuficiência cardíaca direita recorrente após pericardectomia pode ser causada por pericardectomia incompleta, constrição recorrente, disfunção diastólica ou atingimento miocárdico. A identificação de pericardite constritiva (PC) recorrente após pericardectomia é desafiante, uma vez que, muitas das características da constrição podem estar ausentes e não existem ainda guidelines de diagnóstico ou terapêutica para a abordagem desta patologia dada a sua extrema raridade.
Os autores descrevem o caso de um homem, 59 anos de idade, com antecedentes de pericardectomia após PC tuberculosa em 1984, internado para esclarecimento de um quadro clínico dominado por sinais e sintomas de insuficiência cardíaca direita. Após estudo complementar foi diagnosticado PC recorrente. Dada a escassez de casos reportados sobre esta patologia, foram discutidas possíveis abordagens terapêuticas nomeadamente uma segunda pericardectomia, transplante cardíaco e terapêutica médica.
The authors report the case of a 59-year-old man with a history of tuberculosis at age 12, who subsequently developed tuberculous constrictive pericarditis (CP) and underwent pericardiectomy in 1984. The surgical report refers to pericardiectomy by median sternotomy but gives no further details. He also had a history of permanent atrial fibrillation (AF), type 2 diabetes, hypothyroidism and multiple emergency department (ED) admissions for clinical settings interpreted as liver failure; he was medicated with warfarin, bisoprolol 2.5 mg daily, metformin 1000 mg twice daily and levothyroxine 0.1 mg daily. He was admitted to the ED again in September 2013 with abdominal discomfort, peripheral edema and ascites. On physical examination, he presented jugular distension, weak apical pulse, and audible and arrhythmic S1 and S2, with no cardiac murmurs; diminished breath sounds in the lower half of the right hemothorax; palpable hepatomegaly and hepatojugular reflux; and ascites and lower limb edema. He was admitted to the internal medicine department. During hospitalization, he suffered an episode of tight chest pain radiating to both arms, associated with profuse sweating, and he was accordingly transferred to the cardiology department for diagnostic investigation.
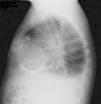
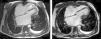
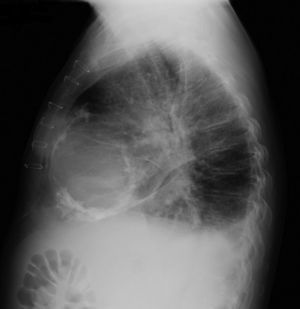
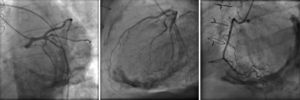
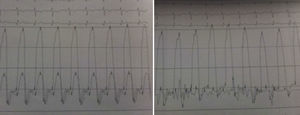
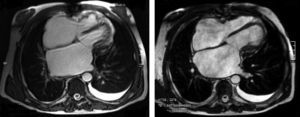
The electrocardiogram revealed AF with a pattern of right bundle branch block, right axis deviation and increased QTc interval (470 ms). Laboratory tests showed hemoglobin 13.0 g/dl, normal renal function and electrolytes, negative serial troponin I, pro-brain natriuretic peptide 313 pg/ml, negative C-reactive protein and procalcitonin, increased alkaline phosphatase (178 U/l) and gamma-glutamyl transpeptidase (109 U/l), normal aspartate aminotransferase, alanine aminotransferase and total bilirubin, reduced total protein (4.6 g/dl) and albumin (1.83 g/dl), normal protein electrophoresis, negative HIV, HBV and HCV, normal anti-neutrophil cytoplasmic antibody, immunoglobulin and complement, and normal thyroid function. Abdominal ultrasound showed an enlarged liver with a diffuse heterogeneous structure, suggesting chronic liver disease, and dilatation of the inferior vena cava and suprahepatic veins. The chest X-ray revealed calcification of the cardiac silhouette and right pleural effusion (Figure 1). Echocardiography showed severe left atrial dilatation (area 44 cm2) and mild right atrial dilatation (25 cm2), while the other chambers and the roots of the great vessels were of normal size; normal left ventricular thickness; altered interventricular septal motion; preserved left ventricular systolic function (ejection fraction 61%); no wall motion abnormalities; diastolic mitral flow compatible with a restrictive pattern (mean E/E′ 19); preserved right ventricular systolic function (tricuspid annular plane systolic excursion 17 mm); no significant valve abnormalities; pulmonary artery systolic pressure (PASP) estimated at 45 mmHg; thickening of the diaphragmatic pericardium due to fibrosis; and dilatation of the inferior vena cava and suprahepatic veins. Coronary angiography revealed no coronary artery disease (Figure 2), but hemodynamic data on catheterization were suggestive of constrictive physiology: dip-and- plateau pattern of the ventricular curve; equalization of ventricular end-diastolic pressures; right curve with prominent y descent; and mild pulmonary hypertension (PASP 45 mmHg) (Figure 3). Magnetic resonance imaging showed foci of hypointense signal in the region of the inferior pericardium due to residual calcifications and an associated ventricular restrictive component, as shown by myocardial hypokinesis on dynamic sequences (Figure 4).
Following intensive diuretic therapy, salt restriction and fluid therapy, the patient presented a favorable clinical course with resolution of heart failure symptoms. The possibility of redo pericardiectomy was discussed with the cardiac surgery team, but it was decided not to perform a repeat procedure (30 years after the first) after weighing the risks and benefits. Heart transplantation was also discussed and the patient underwent respiratory function testing, which revealed moderate obstruction, but the decision was postponed until such time as the patient presented NYHA class III/IV heart failure refractory to medical therapy. He was discharged and referred for cardiology consultation; diuretic therapy was continued and he has remained in NYHA class I/II, with no rehospitalization for decompensation to date. He has also been referred for cardiopulmonary stress testing for further assessment of his functional capacity.
DiscussionCP is an uncommon condition and its diagnosis remains a clinical challenge.1,2 It is defined as the end stage of an inflammatory process involving the pericardium, and virtually any inflammation can cause constriction. In developed countries the main causes of CP are idiopathic or secondary to surgery or radiation.1 Tuberculosis used to be one of the most frequent causes, but the efficacy of antibiotic therapy has reduced the incidence of tuberculous CP in these countries. However, in developing countries it remains one of the most common causes,1,2 and in Africa there is a frequent association between tuberculous CP and HIV-positive status.1,3
Constriction usually develops over a period of years, ultimately resulting in fibrotic thickening of the pericardium, often calcified, with adhesions between the parietal and visceral layers, forming a non-compliant shell around the heart, which restricts diastolic filling of the cardiac chambers.1,2 This leads to elevation and equalization of filling pressures in the chambers and systemic and pulmonary veins. Ventricular filling is abnormally rapid at the beginning of diastole due to elevated atrial pressure and elastic recoil, then ceases abruptly during mid- or end-diastole when the intracardiac volume reaches the limit imposed by the thickened pericardium; this means that practically all ventricular filling occurs at the beginning of diastole, resulting in systemic venous congestion.1,4
Clinical presentation of CP consists mainly of signs and symptoms of right heart failure. The early stage is characterized by peripheral edema, abdominal discomfort and some degree of liver congestion. Disease progression is generally accompanied by ascites, anasarca and jaundice. Signs and symptoms of elevated pulmonary venous pressure may appear, including dyspnea, cough and orthopnea, as well as AF and tricuspid regurgitation. Fatigue and cachexia due to low cardiac output may also be present in more advanced stages.1,2 Occasionally angina occurs due to coronary artery disease or compression of a coronary artery by the thickened pericardium.2
Findings on physical examination include marked elevation of jugular venous pressure with a prominent rapidly collapsing y descent; positive Kussmaul's sign (increased venous pressure on inspiration); paradoxical pulse (fall of >10 mmHg in systolic arterial pressure during inspiration), found in up to a third of patients with CP; weak apical pulse; distant heart sounds; pericardial knock (an early diastolic sound over the left sternal border or apex caused by abrupt cessation of ventricular filling); hepatomegaly, ascites and jaundice; palmar erythema; spider angioma and lower limb edema. As mentioned above, in advanced stages there may be cachexia and muscle wasting.1,2
Differential diagnosis includes pulmonary embolism, chronic obstructive pulmonary disease, liver failure and restrictive cardiomyopathy,5 the latter being the most difficult but also the most important to exclude, since the therapeutic approaches are completely different.
The chest X-ray shows pericardial calcification in a small number of patients, raising the suspicion of tuberculous CP.1,2 Transthoracic electrocardiography may show pericardial thickening, abrupt posterior motion of the interventricular septum at the beginning of diastole (septal bounce), dilatation of the inferior vena cava without inspiratory collapse and of the suprahepatic veins, and predominantly early ventricular filling. Doppler study may document increased transmitral flow during expiration (>25% variation); a restrictive flow pattern; deceleration time <160 ms; increased duration and velocity of pulmonary venous flow with inspiration; or increased diastolic reversal velocity of >25% of forward flow in the suprahepatic veins during expiration.6 Pericardial thickening and calcification may be seen on magnetic resonance imaging.1,2
Cardiac catheterization can document the hemodynamic effects of CP and enable differential diagnosis with restrictive cardiomyopathy. In CP atrial pressures and ventricular diastolic pressure are elevated and equalized (around 20 mmHg). The right atrial pressure curve shows a typical M or W shape resulting from the existence of a preserved descending x wave in systole, a prominent descending y wave in diastole and small a and v waves of the same amplitude. Ventricular diastolic pressure curves show a fall at the beginning of diastole, followed by a plateau (dip-and-plateau pattern). PASP is generally less than 45−50 mmHg. Ejection volume is usually reduced but cardiac output at rest is preserved through compensatory tachycardia until more advanced stages of the disease.1,2
The European Society of Cardiology guidelines recommend pericardiectomy as the only definitive treatment for CP; this may by anterolateral thoracotomy or median sternotomy5 and can be complete or partial. Chowdhury et al. retrospectively compared the surgical results between complete and partial pericardiectomy and found that survival and functional outcome were superior with complete pericardiectomy compared with partial pericardiectomy.7
Complete pericardiectomy is defined as wide excision of the pericardium anteriorly extending to both phrenic nerves and including the diaphragmatic pericardium. Partial pericardiectomy is defined as any pericardial excision that does not meet criteria for phrenic-to-phrenic pericardiectomy.8
Ling et al. studied patients who underwent pericardiectomy at the Mayo Clinic between 1985 and 1995, comparing them with a historic cohort, and found lower perioperative mortality (6% vs, 14%; p=0.011), higher median age at the time of pericardiectomy (61 vs. 45 years), and an increased prevalence of CP due to radiation, but late survival was not as good as expected. The principal causes of perioperative mortality were low output state, sepsis, uncontrolled bleeding, renal failure and respiratory insufficiency. Independent predictors of late survival were age, NYHA class and previous radiation.9
A retrospective study by Bertog et al. of 163 patients who underwent pericardiectomy over a 24-year period concluded that the only predictors of late events were post-radiation etiology, age, left ventricular dysfunction, high pulmonary artery pressure, creatinine and serum sodium.8 George et al. concluded that post-radiation CP, hyperbilirubinemia and hypoalbuminemia were significant risk factors for decreased long-term survival after pericardiectomy.10
Right-sided heart failure after pericardiectomy can be caused by incomplete pericardiectomy, recurrent constriction due to exuberant scar tissue, diastolic dysfunction or extension of pericardial calcification into the myocardium.7,9 Other possible etiologies include cardiomyopathy and pulmonary hypertension.7
Identification of constriction in patients who have recurrent symptoms after partial pericardiectomy is challenging, since the characteristic Doppler echocardiographic features may not be present if a portion of the ventricles is free from constricting pericardium. Furthermore, many of these patients are under intensive diuretic therapy and/or have underlying cardiac disease, such as coronary artery disease, that may mask the signs and symptoms. Magnetic resonance imaging and cardiac catheterization may be required to establish the diagnosis.7
One possible approach for recurrent CP following pericardiectomy is repeat pericardiectomy, although the risks and benefits of a second operation are not well established. Cho et al. analyzed the outcomes of second pericardiectomy in 41 patients hospitalized for recurrent CP between 1993 and 2010. They divided the study population into two groups according to the interval between the first and second operations (≤1 year vs. >1 year), and found that five-year survival was significantly better in the group with a shorter interval between operations (73% vs. 29%, p=0.032). Multivariate analysis showed that NYHA class was also an important predictor of survival.7 The authors stressed the importance of complete resection at first operation given the significant mortality associated with repeat pericardiectomy; at the same time, the poor clinical outcome of late (more than 1 year) reoperation may be explained by progressive diastolic dysfunction or myocardial involvement.7
In conclusion, CP is a heterogeneous disease, increasingly important causes of which in the current era include radiation and cardiac surgery. Although pericardiectomy is often performed and the results are excellent in some patients, it may not offer a cure or good long-term results in advanced or post-radiation CP.9 It is therefore essential to investigate other treatment options, particularly heart transplantation, which should be considered in selected patients without recurrent tumor and with good pulmonary reserve.9
CP is rare, and recurrent CP rarer still, and there are as yet no diagnostic or treatment guidelines for management of the disease. There is thus a need to establish the best approach in these patients, for which further studies and much research will be required.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ferreira R, Gonzaga A, Santos L, et al. Pericardite constritiva recorrente – um desafio diagnóstico e terapêutico. Rev Port Cardiol. 2015;34:421.e1–421.e5.