Venous air embolism (VAE) is an entity with potential for severe morbidity and mortality.1 It is predominantly an iatrogenic complication of invasive diagnostic and therapeutic maneuvers.2
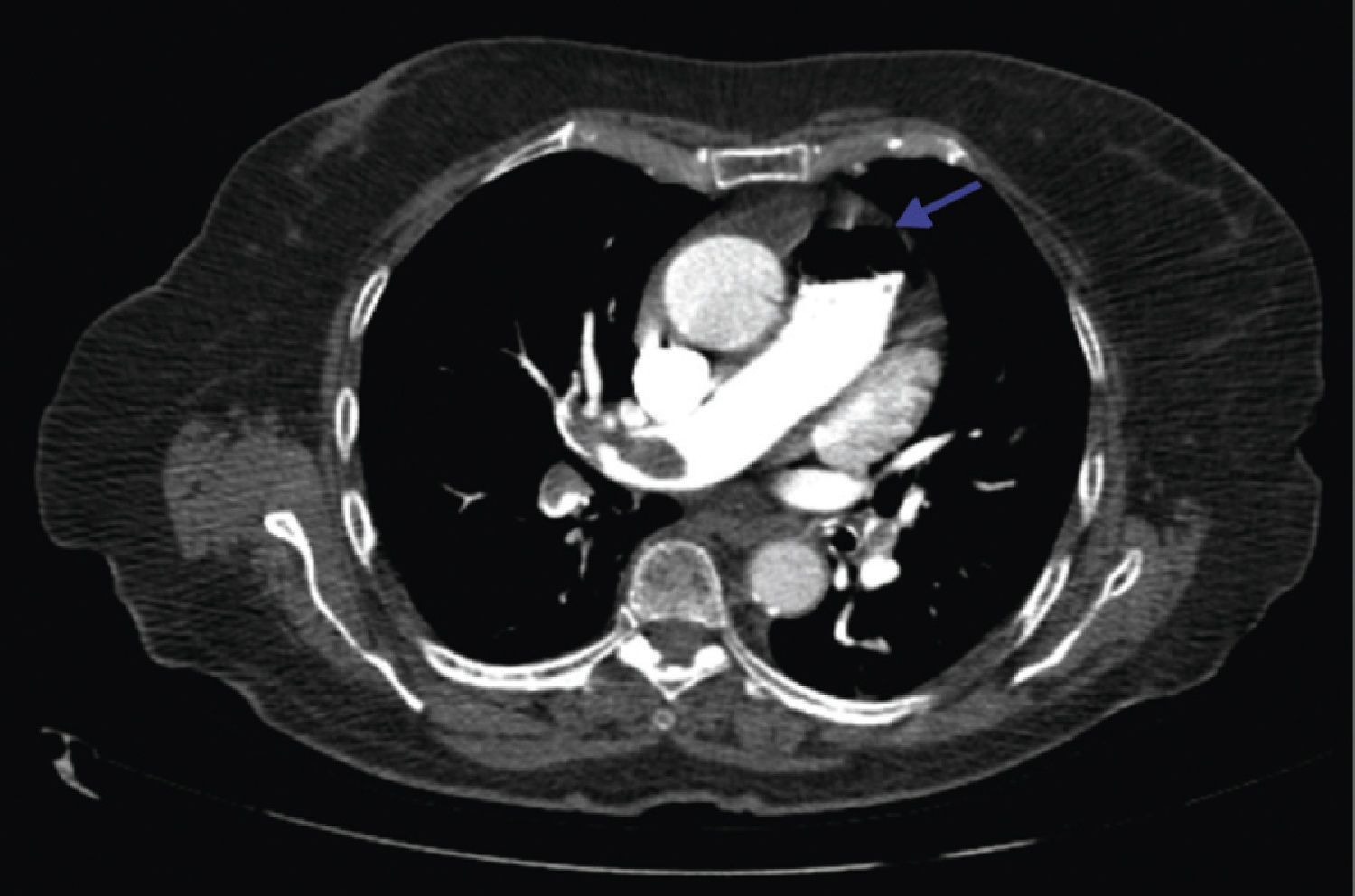
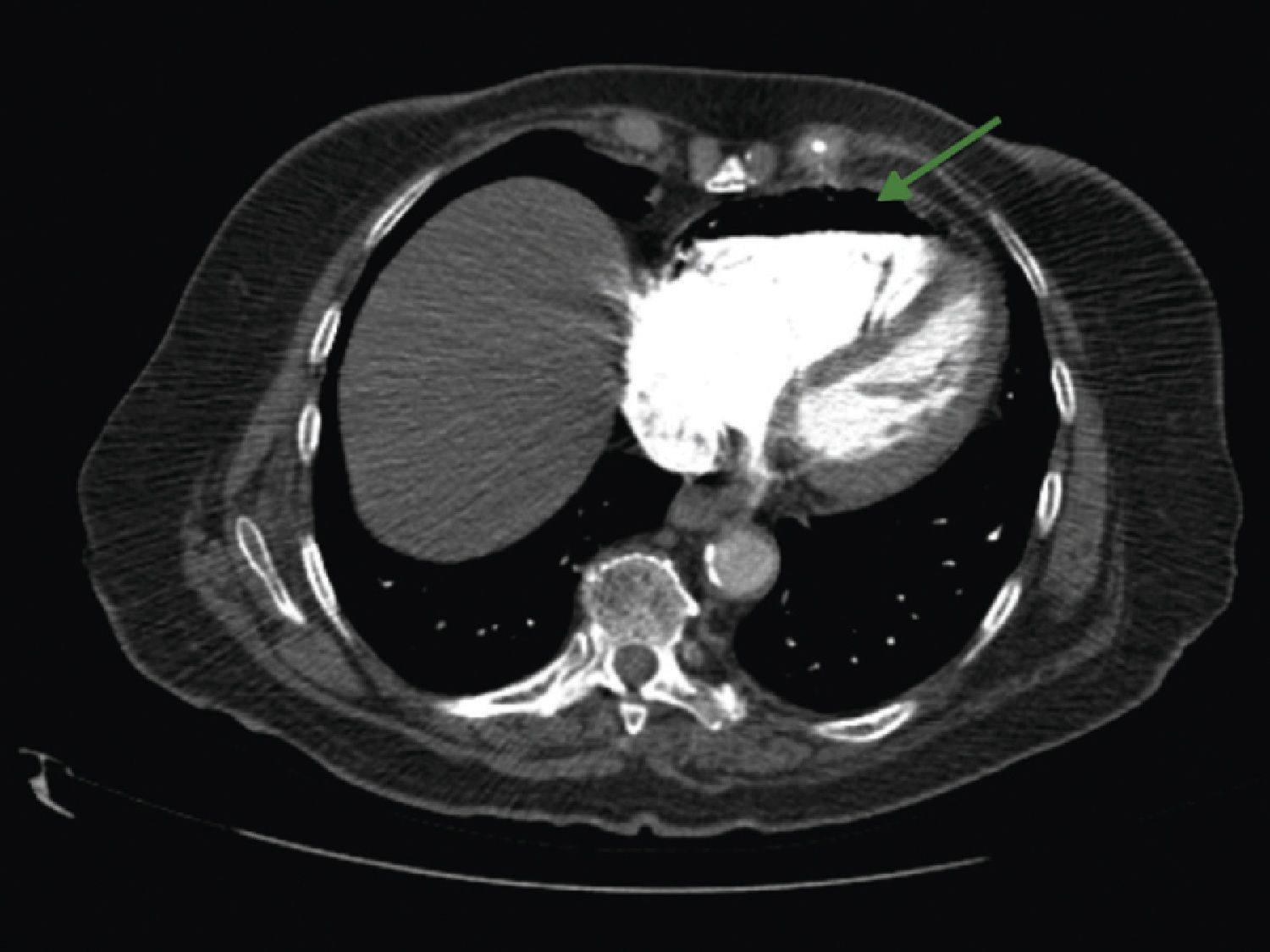
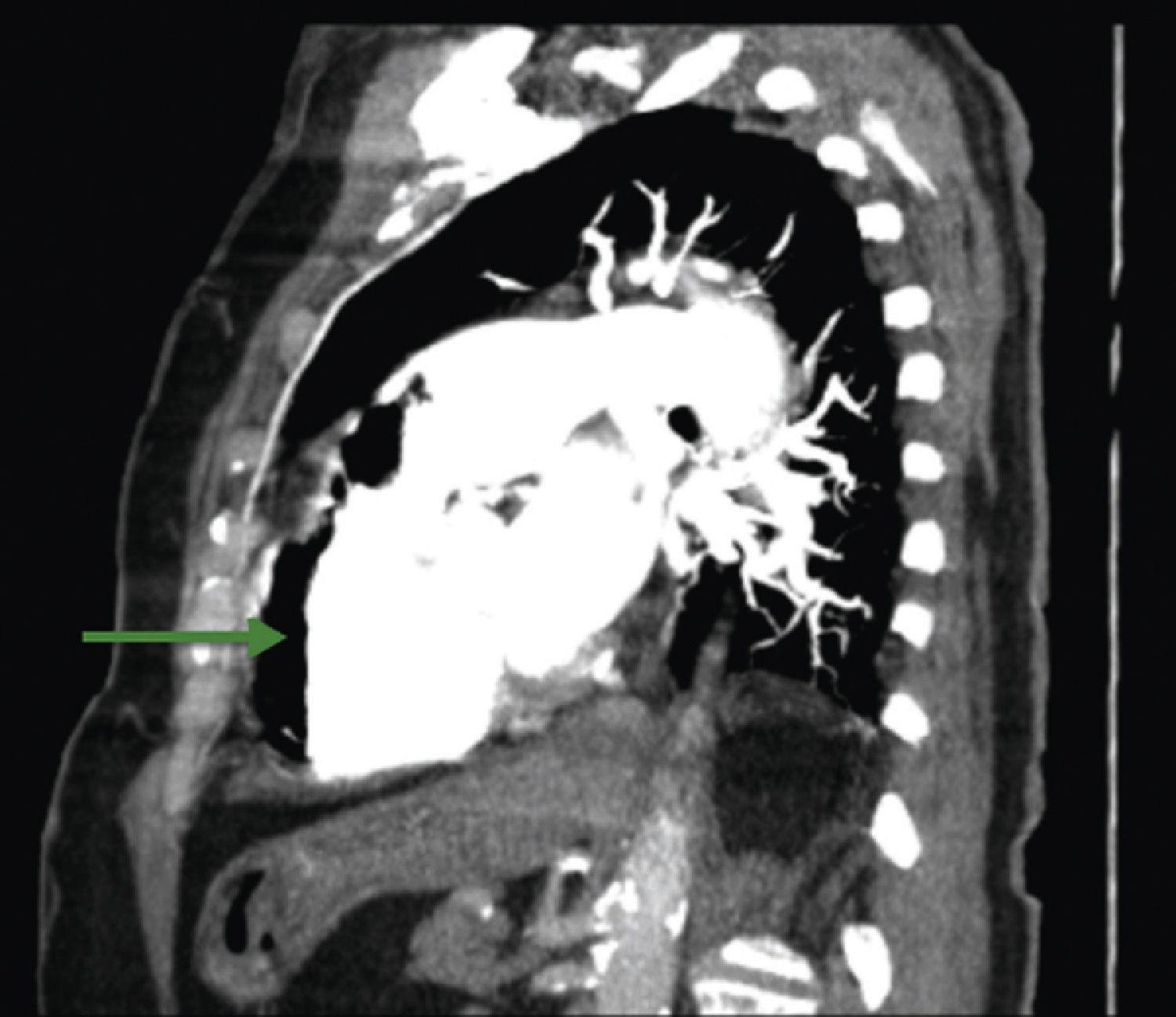
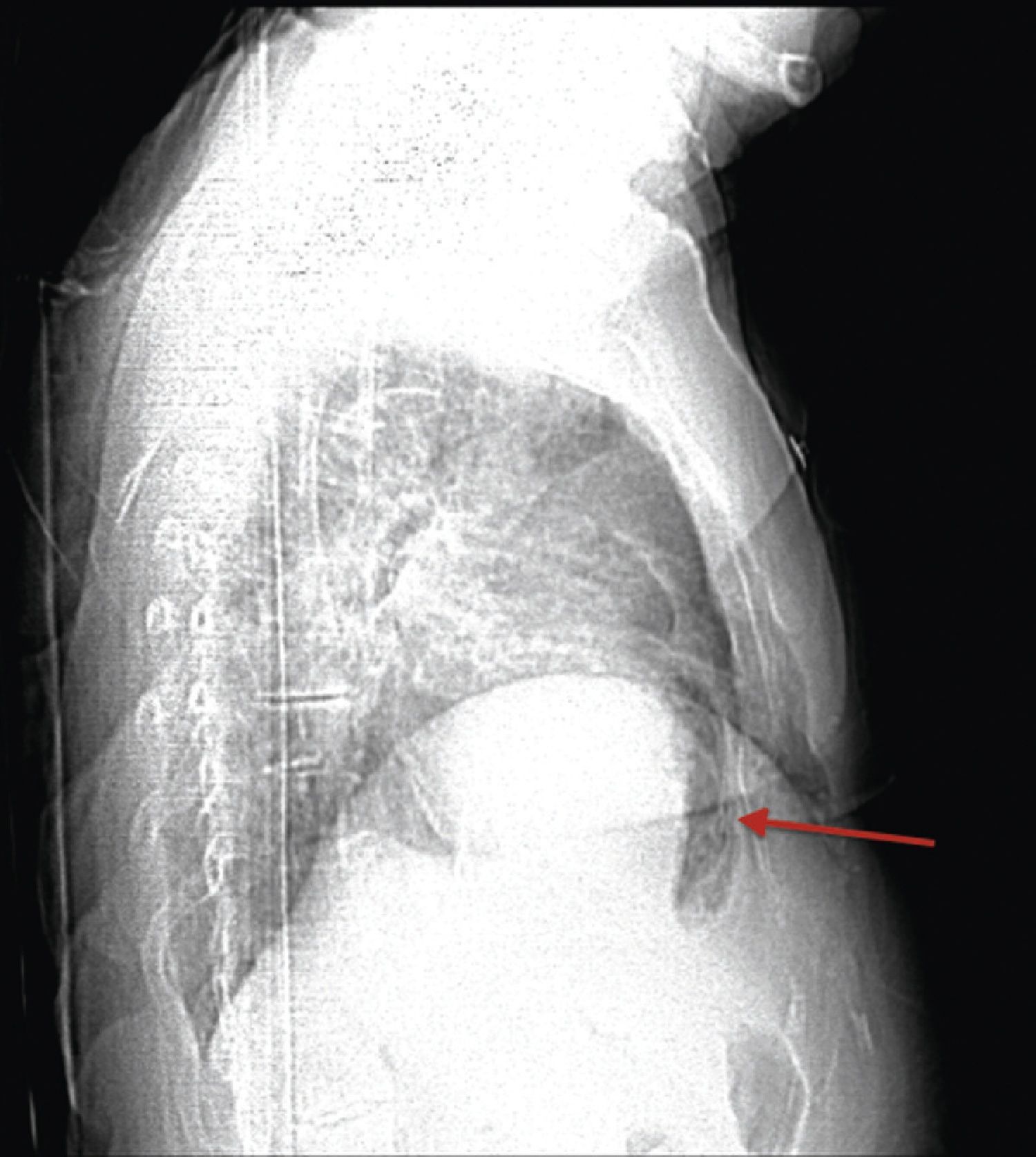
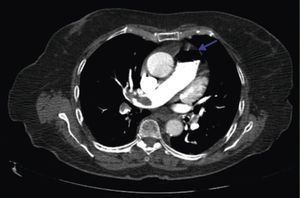
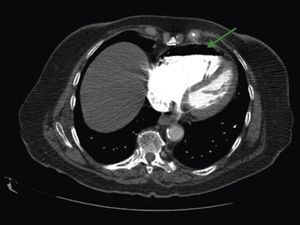
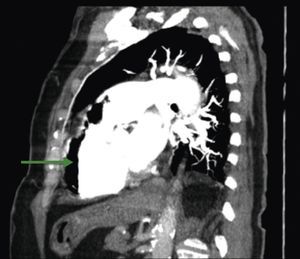
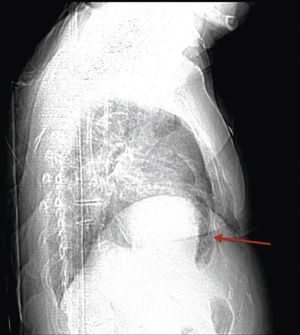
A 84‐year‐old woman was referred to our emergency department from another hospital with acute dyspnea at rest and hypoxemia. On admission a peripheral venous catheter had been inserted for fluid replacement and she had undergone computed tomography pulmonary angiography (CTPA) for suspected pulmonary embolism. Soon after the exam the patient became confused and drowsy and her respiratory failure worsened significantly without hemodynamic instability. CTPA confirmed the presence of bilateral central and peripheral thrombus and a large amount of air in the brachiocephalic veins, pulmonary artery (Figure 1) and right ventricle (Figures 2 and 3). She was considered to have VAE unrelated to the administration of contrast, given a topogram image that suggested the presence of air in the right ventricle before contrast injection (Figure 4). It was therefore assumed that the VAE resulted from the placement or manipulation of the peripheral venous catheter previously inserted in another institution. The patient was immediately given normal saline, a Hudson mask was applied and she was placed in left lateral decubitus position (Durant's maneuver) and in the Trendelenburg position, to keep the right ventricular outflow tract lower than the right ventricular cavity causing the air to migrate upwards, minimizing the likelihood of embolism. Her clinical status improved rapidly and she recovered without sequelae. Bedside transthoracic echocardiography showed no acute ventricular dilation or dysfunction, and the patient was transferred to another hospital for hyperbaric oxygen therapy.
The patient returned to our hospital after two days, and was discharged home three days later.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.