Increased left atrial (LA) size is a prognostic marker of mortality in the general population. LA size varies considerably in patients with dilated cardiomyopathy (DCM), but its clinical significance has not been widely studied.
ObjectiveTo evaluate the long-term prognostic value of LA volume (LAV) in patients with DCM.
MethodsWe prospectively studied patients admitted between January and December 2004 with a diagnosis of DCM, in sinus rhythm. Complete echocardiographic study at rest and after pharmacological stress was performed in all patients.
The composite endpoint of mechanical ventricular assistance (MVA), heart transplantation or death during follow-up was assessed by univariate and multivariate analysis using a Cox regression model.
ResultsThe study population consisted of 35 patients (68.6% male, mean age 52.0) with DCM, 82.9% of non-ischemic etiology. Ejection fraction (EF) at rest was 31.1±9.4%.
During follow-up, eight patients died, one was placed on MVA and one underwent transplantation. Univariate Cox analysis showed various potential echocardiographic markers of prognosis in our population, including LA size in M-mode (HR 1.12, CI: 0.99–1.26, p=0.067), LAV (HR 1.03, CI: 1.00–1.07, p=0.046), LAV adjusted for body surface area (HR 1.03, CI: 0.99–1.26, p=0.049), E/A ratio (HR 0.99; CI: 0.99–1.81; p=0.060); E/A >2 (HR 7.00, CI: 1.48–32.43, p=0.014) and mitral E/E’ ratio (HR 1.04, CI: 1.00–1.09, p=0.074).
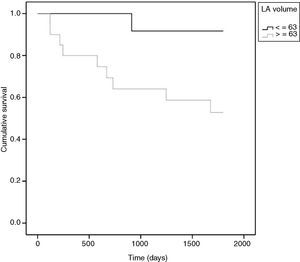
The only variable that remained in the multivariate model was LAV, with a cut-off value of 63 ml (HR 7.7, CI: 0.97–60.61, p=0.05).
ConclusionsLAV was the only echocardiographic determinant of MVA, heart transplantation or death in our population with DCM. The echocardiographic parameters commonly used for risk stratification such as EF, left ventricular end-diastolic diameter and contractile reserve did not show prognostic significance in our study.
O aumento da aurícula esquerda (AE) é um marcador de mortalidade na população geral. Os doentes com miocardiopatia dilatada (MCD) têm um amplo espetro de tamanhos de AE, mas a importância clínica desta observação tem sido pouco estudada.
ObjectivoAvaliar a importância prognóstica a longo prazo do volume da AE (VAE) em doentes com MCD.
MétodosEstudo prospetivo de doentes admitidos durante o ano de 2004 com o diagnóstico de MCD, em ritmo sinusal. Foi realizado estudo ecocardiográfico completo em repouso e após stress farmacológico. O endpoint composto considerou a assistência ventricular mecânica (AVM), a transplantação cardíaca ou a morte.
ResultadosForam incluídos 35 doentes (68,6% sexo masculino, idade média 52,0), 82,9% etiologia não isquémica. Fração ejeção em repouso 31,1 ± 9,4%.
Durante o seguimento, oito doentes morreram, um foi colocado em AVM e um foi transplantado. A análise de Cox univariável revelou potenciais marcadores ecocardiográficos de prognóstico na amostra tais como a dimensão da AE em modo M (HR-1,12; IC: 0,99-1,26; p = 0,067); VAE (HR-1,02; IC: 1,00-1,04; p = 0,046); VAE ajustado à superfície corporal (HR-1,03; IC: 1,00-1,07; p = 0,049); E/A (HR-0,99; IC: 0,99-1,81; p = 0,060); E/A > 2 (HR-7,00; IC:1,48-32,43; p = 0,014) e E/E’ mitral (HR-1,04; IC: 1,00-1,09; p = 0,074). Na análise multivariável a única variável que permaneceu no modelo foi o VAE com o ponto de corte de 63 ml (HR-7,7, IC: 0,97-60,61, p = 0,05).
ConclusãoNesta amostra, o VAE foi o único parâmetro ecocardiográfico determinante de AVM, transplantação cardíaca ou morte. Os parâmetros ecocardiográficos habitualmente utilizados para estratificação de risco, tais como a fração ejeção do ventrículo esquerdo, a dimensão do ventrículo esquerdo e a reserva contrátil não tiveram valor prognóstico na nossa amostra.
Dilated cardiomyopathy (DCM) is characterized by left ventricular (LV) dilatation and systolic dysfunction without a chronic increase in afterload (as in aortic stenosis or hypertension) or volume overload (as in mitral regurgitation). Historically, the prognosis of DCM patients was dismal, with mean survival of two years after diagnosis.1 Despite advances in medical, interventional and surgical treatment over the last twenty years, the disease still has an extremely poor long-term prognosis.
In patients with suspected heart failure and LV dysfunction, echocardiography is the most important diagnostic exam for establishing the diagnosis and for assessing the presence and severity of LV dilatation and dysfunction. Diagnostic criteria include reduced ejection fraction (EF) (<40%) and increased LV end-diastolic diameter (LVEDD) (>35 mm/m2). Besides diagnosis, echocardiography is also important for determination of etiology when possible and for risk stratification (Table 1).
Echocardiographic markers of prognosis in patients with dilated cardiomyopathy.2
| Prognostic marker | Echocardiographic parameters |
| LV size | LVEDD, LVEDV |
| LV systolic function | Ejection fraction |
| LV diastolic function | Pseudonormal or restrictive pattern |
| RV function | TAPSE |
| Pulmonary hypertension | Tricuspid regurgitation velocity |
| Left atrial size | Left atrial volume |
| Mitral regurgitation | Presence, severity and mechanism |
| Contractile reserve | As determined by stress echocardiography |
LV: left ventricular; LVEDD: left ventricular end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; RV: right ventricular; TAPSE: tricuspid annular plane systolic excursion.
Increased left atrial volume (LAV) is associated with poor prognosis in many forms of cardiovascular disease, including hypertension, hypertrophic cardiomyopathy and heart failure, as well as with non-cardiac diseases, such as end-stage renal failure requiring hemodialysis.3 In one study, LAV was an independent predictor of cardiovascular death, heart failure, atrial fibrillation and stroke in an unselected population of 483 individuals in sinus rhythm, in a mean follow-up of seven years.4 However, its prognostic value in patients with DCM has not been widely studied.
In the absence of mitral valve disease or atrial fibrillation, left atrial (LA) dilatation is due to increased LV diastolic pressures, reflecting hemodynamic status.5 Patients with impaired systolic function present higher diastolic pressures, leading to LA overload and progressive dilatation. Thus, the degree of LA dilatation reflects the duration and severity of LV dysfunction.6
Quantification of left atrial sizeThe left atrium acts as a contractile pump that delivers 15–30% of LV diastolic filling.7 It should be measured at end-systole, at its maximum size. Planimetric images should be obtained showing clear contours in apical 4- and 2-chamber views. The confluence of the pulmonary veins and the left atrial appendage should be excluded. Transesophageal echocardiography often fails to provide a view of the LA that gives a reliable assessment of its size.8
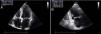
LA size can be assessed in various ways, but M-mode or two-dimensional derived anteroposterior linear dimension obtained in parasternal long-axis view is the standard for linear measurement. Although these linear measurements correlate with angiographic measurements and are widely used in clinical practice and research, they do not accurately represent true LA size.9 As the LA is not spherical and dilates asymmetrically, increasing emphasis is now placed on LAV rather than LA diameter, since the latter does not reflect longitudinal dilatation. In addition, the association between LA size and cardiovascular disease is stronger for LAV than for its linear dimension.5 The simplest method for estimating LAV is the cube formula, which assumes the LA is in fact spherical, but this has proved inferior to other techniques using an ellipsoid model or Simpson's rule (Figure 1). Since 2005 the American Society of Echocardiography (ASE) has recommended use of the latter two methods in clinical practice.8
ObjectiveThe aim of this study was to evaluate the long-term prognostic value of LAV in patients with DCM.
MethodsThis was a cohort study of patients admitted for heart failure in 2004 with a diagnosis of DCM (ischemic or non-ischemic), in sinus rhythm and in New York Heart Association (NYHA) functional class
All patients underwent complete echocardiographic study at rest and after pharmacological stress, cardiopulmonary exercise test (CPET) and NT-proBNP measurement.
Echocardiographic assessment was performed using a Vivid 7 Dimension scanner (GE Healthcare, Horten, Norway).
The following echocardiographic parameters were assessed: LA diameter in M-mode, LV end-diastolic (LVEDD) and end-systolic (LVESD) diameters, LV end-diastolic (LVEDV) and end-systolic (LVESV) volumes, EF calculated by Simpson's method, LV inflow tract E and A velocities and E/A ratio, and mean E’ velocity by tissue Doppler. LAV was calculated using Simpson's rule in accordance with the ASE guidelines.8 Stress echocardiography was performed with dobutamine (10–40 μg/kg/min) following the protocol used in our institution and in accordance with the European Society of Cardiology guidelines. Final stress EF was determined and patients with a ≥20% increase in EF were considered to have contractile reserve.10 All echocardiographic exams were performed and LAV was calculated by the same operator (AG).
CPET was performed on a treadmill using the modified Bruce protocol on a SensorMedics Vmax 229 system (Yorba Linda, Calif.). Oxygen uptake (VO2), carbon dioxide output (VCO2) and minute ventilation (VE) were measured cycle by cycle. Peak oxygen uptake (pVO2), percentage of predicted pVO2 (%pVO2) and VE/VCO2 slope (VE/VCO2) were analyzed. pVO2 was defined as mean VO2 in the last 30 seconds of the test; VE/VCO2 was calculated by the system.
NT-proBNP was measured using Roche Elecsys electrochemiluminescence immunoassay (pg/ml).
Information on patient follow-up was obtained from medical records or by telephone contact in cases of missing data. The composite endpoint was need for mechanical ventricular assistance (MVA), heart transplantation or death (adverse events).
Statistical analysisContinuous variables are expressed as means ± standard deviation or medians and 25th (P25) and 75th (P75) percentiles, as appropriate, and categorical variables as percentages, and compared using the Mann-Whitney exact test and chi-square test.
A Cox regression model was used to analyze survival. Variables with p<0.15 in univariate analysis were included in the multivariate model. Applicability of the Cox regression model (proportional hazards) was confirmed using a formal test based on scaled Schoenfeld residuals. An LAV cut-off was determined by analysis of the martingale residuals obtained from the Cox regression model. The Kaplan-Meier estimator and the log-rank test were used to compare survival in the groups defined according to this cut-off.
The area under the receiver operator characteristic (ROC) curve was used to determine the discriminatory power of LAV.
A level of α=0.05 was considered significant. Statistical analysis was performed using SPSS, version 19 (SPSS Inc., Chicago, Ill) and R version 2.14.1 (R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
ResultsThe study population consisted of 35 patients (26 male, mean age at admission 52±11 years) with DCM, 17% of ischemic and 83% of non-ischemic etiology; 34% presented complete left bundle branch block, and 6% were in NYHA functional class I, 43% in class II and 51% in class III at the time of enrollment in the study. During follow-up, 48% of patients were implanted with a cardiac resynchronization therapy device or implantable cardioverter-defibrillator. All patients were under optimized medical therapy with beta-blockers and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. The parameters assessed at admission are presented in Tables 2 and 3.
Echocardiographic parameters at admission (n=35).
| Variable | Mean ± SD |
| LA size in M-mode (mm) | 46.6±5.7 |
| LAV (ml) | 82.7±34.9 |
| LAV/BSA (ml/m2) | 44.8±18.8 |
| LVEDD (mm) | 73.5±10.0 |
| LVESD (mm) | 58.9±11.1 |
| LVEDV (ml) | 214.1±82.9 |
| LVESV (ml) | 153.0±76.0 |
| EF (%) | 31.1±9.4 |
| E/A | 2.08±1.52 |
| E/A >2, n (%) | 15 (43%) |
| E/E’ | 17.9±10.9 |
| MR area (cm2) | 4.0±3.36 |
| PASP (mmHg) | 44.0±13.7 |
| Stress EF (%) | 38.0±10.9 |
| CR, n (%) | 21 (60%) |
BSA: body surface area; CR: contractile reserve; MR: mitral regurgitation; PSAP: pulmonary artery systolic pressure. Other abbreviations as in text.
Ten adverse events were recorded in a median follow-up of 60 months (minimum 4 months; maximum 60 months): eight patients died, one was placed on MVA and one underwent transplantation. Complete 60-month follow-up was achieved in 20 patients, with a median of 43.6 months (minimum 10.2; maximum 48.7) in the other five. The characteristics of the study population at admission divided into those with and without events are shown in Table 4.
Demographic, echocardiographic and cardiopulmonary characteristics and NT-proBNP measurements in those with and without events.
| Without events (n=25) | With events (n=10) | p | |
| Male, n (%) | 17 (68%) | 7 (70%) | 0.908** |
| Age | 50.3±10.4 | 56.1±10.9 | 0.174* |
| LBBB, n (%) | 8 (32%) | 4 (40%) | 0.650** |
| LA size (mm) | 45.6±6.0 | 49.09±4.6 | 0.109* |
| LAV (ml) | 75.2±34.25 | 100.0±31.4 | 0.038* |
| LAV/BSA (ml/m2) | 41.1±18.0 | 54.1±18.4 | 0.045* |
| LVEDD (mm) | 73.8±9.7 | 72.9±11.3 | 0.956* |
| LVESD (mm) | 59.0±10.3 | 58.9±13.4 | 0.956* |
| LVEDV (ml) | 209.7±75.5 | 225.0±102.8 | 0.661* |
| LVESV (ml) | 147.5±65.0 | 166.9±101.1 | 0.742* |
| EF (%) | 32.1±8.1 | 28.8±12.3 | 0.303* |
| EF <35%, n (%) | 13 (52%) | 7 (70%) | 0.331** |
| E/A | 1.73±1.45 | 2.97±1.38 | 0.006* |
| E/A >2, n (%) | 7 (28%) | 8 (80%) | 0.005** |
| E/E’ | 15.5±9.9 | 24.0±11.7 | 0.022* |
| Area of MR (cm2) | 3.65±3.58 | 4.73±2.89 | 0.418 |
| PASP (mmHg) | 48.43±15.40 | 38.60±9.70 | 0.207 |
| Stress EF (%) | 39.9±10.3 | 33.3±11.6 | 0.111* |
| CR, n (%) | 16 (64%) | 5 (50%) | 0.440** |
| NT-proBNP (pg/ml) (median P25-P75) | 1051 (381–2302) | 5543 (1419–7041) | 0.005* |
| pVO2 (ml/kg/min) | 22.7±5.1 | 14.8±2.9 | <0.001* |
| % predicted pVO2 | 72.5±16.0 | 54±16.9 | 0.007* |
| VE/VCO2 | 32.5±7.2 | 41.1±7.1 | 0.008* |
| Follow-up | 60 (P25-60; P75-60) | 20.8 (P25-6; P75-33) | NA |
BSA: body surface area; CR: contractile reserve; MR: mitral regurgitation; PASP: pulmonary artery systolic pressure. Other abbreviations as in text.
The potential echocardiographic markers of prognosis identified by univariate Cox analysis (Table 5) were LAV, E/A, LA size and E/E’.
Results of Cox univariate analysis of echocardiographic parameters.
| HR | CI | p | |
| LA size (mm) | 1.12 | 0.99–1.26 | 0.067* |
| LAV (ml) | 1.02 | 1.00–1.04 | 0.046* |
| LAV/BSA (ml/m2) | 1.03 | 1.00–1.07 | 0.049** |
| LVEDD (mm) | 0.99 | 0.93–1.05 | 0.734 |
| LVESD (mm) | 1.00 | 0.94–1.06 | 0.955 |
| LVEDV (ml) | 1.00 | 0.99–1.01 | 0.696 |
| LVESV (ml) | 1.00 | 0.99–1.01 | 0.327 |
| EF (%) | 0.97 | 0.90–1.04 | 0.371 |
| E/A | 0.99 | 0.99–1.81 | 0.060* |
| E/A >2 | 7.00 | 1.48–32.43 | 0.014*** |
| MR area (cm2) | 1.07 | 0.91–1.25 | 0.405 |
| PASP (mmHg) | 0.947 | 0.86–1.04 | 0.248 |
| E/E’ | 1.04 | 1.00–1.09 | 0.074* |
| CR | 1.64 | 0.47–5.68 | 0.434 |
| pVO2 | 0.68 | 0.57–0.82 | <0.001**** |
| NT-proBNP/100 | 1.01 | 1.00–1.02 | 0.038**** |
BSA: body surface area; CR: contractile reserve; MR: mitral regurgitation; PASP: pulmonary artery systolic pressure. Other abbreviations as in text.
In Cox multivariate analysis, the only echocardiographic variable that remained in the model was LAV. The data in Table 5 show that each 1-ml increment in LAV increases risk for an adverse event by 2%, each 5-ml increment in LAV increasing risk for adverse events by 9.2% (HR: 1.092; CI: 1.00–1.19, p=0.046).
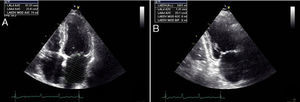
For a clearer picture of the clinical implications, a cut-off of 63 ml for LAV was determined (Figure 2). Patients with LAV >63 ml had a higher risk for adverse events (p=0.023) (Figure 3). The risk for adverse events was quantified as HR 7.7 (CI 0.97–60.61, p=0.05), meaning that the risk for an adverse event was 7.7 times higher in those with LAV >63 ml.
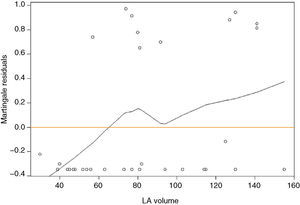
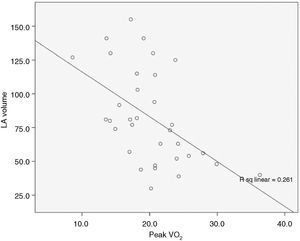
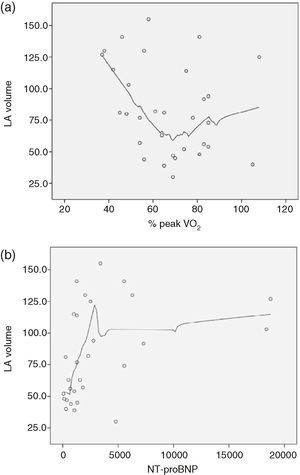
There was also a correlation between LAV and pVO2, with Spearman's correlation coefficient of −0.55, p=0.001 (Figure 4). We also sought to correlate LAV with other known prognostic markers in DCM such as %pVO2 and NT-proBNP, but the relationship between these variables is not linear, and so it was not possible to determine a correlation coefficient (Figure 5A and B, respectively).
DiscussionThis cohort study of patients with DCM with a mean follow-up of five years showed that LAV is an echocardiographic prognostic marker of MVA, heart transplantation or death. Most physicians and researchers have focused on EF, LV size and contractile reserve to estimate severity and prognosis in DCM. This prospective study with a relatively long follow-up confirmed that LAV is an independent prognostic marker in DCM that is more powerful than other more commonly used echocardiographic parameters.
The prognostic impact of LAV in patients with LV dysfunction was first described in a post hoc analysis of the SOLVD trials,11 in which the risk for events was proportional to LAV, independently of EF, age and symptomatic status.
Rossi et al. have published two studies12,13 assessing the prognostic value of LAV in patients with LV dysfunction: the first, in 2002, of 337 patients with DCM, with a mean follow-up of 41 months, and the second, in 2007, of 273 patients with heart failure and LV dysfunction (EF <50%), with a mean follow-up of 45 months. We found no other studies in the literature designed with the same objective. Since our population was more similar to that of the 2002 Rossi study,12 which had a composite endpoint of heart transplantation or death, it is more suitable for comparison with our results. The population analyzed by Rossi et al. differed from ours in that it included patients with atrial fibrillation and that the proportion of patients with DCM of ischemic etiology was significantly higher (75%). Mean EF was similar in the two studies, but Rossi et al. did not present data on contractile reserve or other parameters to characterize disease severity (such as pVO2 or NT-proBNP). The incidence of adverse events was similar in the two studies (28% vs. 25%). Rossi et al. recorded 84 adverse events during follow-up, and found various potential clinical and echocardiographic markers of prognosis. LAV and LAV adjusted to body surface area were more powerful predictors of survival than EF, LVESD, E/A ratio, restrictive mitral filling pattern or severity of mitral regurgitation.
Preliminary results on our patient cohort were published in 2009,14 in which the composite endpoint was hospitalization for heart failure, death or heart transplantation. The present article is a continuation of that study, with a further three years of follow-up, and a composite endpoint of death, heart transplantation and MVA. Since the follow-up was significantly longer, the statistical methodology used was also different, to take account of the timing of events.
The main value of our study lies in the long follow-up (complete in 30 patients) and thorough functional evaluation of patients, including CPET, NT-proBNP and contractile reserve. The latter is often used as a prognostic marker in DCM15 but it is time-consuming and requires specialist training. The study's main limitation is its small sample size, a consequence of the time need for such a comprehensive functional evaluation in a relatively uncommon disease. Since this was a study of effectiveness, all assessments were performed in the context of our department's everyday clinical practice; however, to minimize the inevitable interobserver variability of echocardiographic assessment, all exams were performed by the same operator.
The parameters most widely used in clinical practice to assess prognosis in DCM, such as EF, contractile reserve and LV volumes, did not show the expected prognostic significance, even in univariate analysis. The predictive ability of LAV was independent and stronger than that of other echocardiographic parameters used to assess diastolic function (E/A and E/E’), but weaker than pVO2 and NT-proBNP. The LAV cut-off of 63 ml as a marker of prognosis was lower than that obtained in the study by Rossi et al. This may be explained by the characteristics of the two populations, since their study included patients with permanent atrial fibrillation, which was an exclusion criterion in our study in order to avoid confounding factors. The method of assessing LAV was also different, Rossi et al. having used the biplane area-length method, which is the least rigorous of the three usual methods and the one that generally gives higher LAV values.16
In patients with heart failure due to DCM, diastolic dysfunction is an important marker of disease severity. Various studies have demonstrated that degree of diastolic dysfunction shows a stronger correlation with symptoms and prognosis than EF.17 The importance of LA size as an indicator of diastolic function is well known, and LAV is the most useful echocardiographic parameter in this respect. However, the time required for its assessment means it is little used in clinical practice. The latest ASE guidelines recommend LAV assessment by the biplane method of disks (modified Simpson's rule),8 which has good reproducibility compared to magnetic resonance imaging and three-dimensional echocardiography,18 and so this was the method chosen in our study.
LA size has been shown to correlate with prognosis in patients with other forms of cardiovascular disease in which diastolic dysfunction is a major component, including aortic stenosis, restrictive cardiomyopathy and hypertrophic cardiomyopathy.19–21
ConclusionLAV was shown to be an echocardiographic determinant of MVA, heart transplantation or death in our population with DCM. Other echocardiographic parameters commonly used for risk stratification did not show prognostic significance.
The results of this study suggest that LAV assessment, which can be performed using echocardiography, a non-invasive, readily available and inexpensive imaging modality, contributes to risk stratification and should be a routine part of echocardiographic study in patients with DCM.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank Marta Alves for her help with the statistical analysis of data.
Please cite this article as: Ferreira F, Galrinho A, Soares R, et al. O volume da aurícula esquerda como marcador ecocardiográfico de prognóstico em doentes com miocardiopatia dilatada. Rev Port Cardiol. 2013;32:865–872.