Pulmonary embolism (PE) is a common cardiovascular emergency that, when combined with chronic thromboembolic pulmonary hypertension (PH), is associated with high mortality and morbidity. We aimed to determine the incidence of and predisposing factors for the development of PH after a PE episode.
MethodsA retrospective study was conducted in 213 patients admitted to an intensive care unit with intermediate-to-high risk PE between 2000 and 2010. Clinical data at admission were collected and the incidence of PH as assessed by echocardiography (estimated pulmonary systolic artery pressure over 40 mmHg) was determined. Multivariate analysis was used to determine predictors of development of PH.
ResultsPH was detected in 12.4% of patients after a mean follow-up of three years. Only age (hazard ratio [HR] 1.09, 95% confidence interval [CI] 1.02–1.20 per year; p=0.012) and body mass index (HR 1.19, 95% CI 1.04–1.36) per kg/m2, p=0.013) emerged as independent predictors of the development of this complication during follow-up.
ConclusionsPH after PE was a relatively common complication in our series. We identified advanced age and increased body mass index as predisposing factors.
A tromboembolia pulmonar (TEP) é uma emergência cardiovascular comum que, juntamente com a hipertensão pulmonar tromboembólica crónica (HPTEC), se associa a alta mortalidade e morbilidade. Não há ainda consenso quanto à consecutividade destas patologias. Este estudo pretende determinar a incidência e fatores de risco para o desenvolvimento de hipertensão pulmonar (HTP) após uma TEP de risco intermédio a elevado.
MétodosEstudámos retrospetivamente 213 doentes com TEP de risco intermédio a elevado, entre 2000–2010, e comparamos dados demográficos, clínicos, laboratoriais e imagiológicos entre os doentes que manifestaram HTP durante o seguimento clínico (doentes que, pelo menos três meses após a alta, mantivessem pressões sistólicas na artéria pulmonar acima de 40 mmHg, estimadas por ecocardiografia) com os doentes que mantiveram pressões normais.
ResultadosIdentificou-se HTP após a TEP em 12,4% dos doentes. Foram fatores preditores para o desenvolvimento desta complicação a idade (hazard ratio [HR] 1,09 [intervalo de confiança {IC} 95% 1,02–1,20] por ano de idade, p=0,012) e o índice de massa corporal (HR 1,19 [IC 95% 1,04-1,36] por kg m−2, p=0,013).
ConclusõesA HTP após uma TEP foi uma complicação relativamente comum na nossa série de doentes com um episódio agudo de TEP de risco intermédio a elevado. Identificamos como fatores preditores do desenvolvimento desta complicação a idade avançada e a obesidade.
Pulmonary embolism (PE) is a common cardiovascular emergency that is characterized by occlusion of the pulmonary artery lumen by an embolus, which can lead to potentially fatal but reversible right ventricular failure.1 Mortality from PE is around 12/10000 per year,2–4 reaching 60% for acute episodes.5
Clinical presentation at admission is used to stratify patients for short-term risk of death. Those in shock or hypotensive are considered at high risk, those with evidence of right ventricular dysfunction and/or myocardial damage at intermediate risk, and those without shock, hypotension, right ventricular function or markers of myocardial damage at low risk.6 Anticoagulation should be instituted even before the diagnostic process is complete in cases of high or intermediate probability of PE, while fibrinolysis should be reserved for high-risk patients (class I recommendation) and for certain intermediate-risk patients (class IIb recommendation), in the absence of contraindications.6 Fi-brinolysis has been shown to produce a rapid decrease in perfusion defects and right ventricular dysfunction, but does not reduce mortality in intermediate-risk patients.7
While in most patients pulmonary artery occlusion resolves within 6–12 months, development of chronic thromboembolic pulmonary hypertension (CTEPH) can be an extension of the natural history of acute PE.7 However, there is no consensus on its incidence, reports ranging between 0.6% and 7.0%.7–11 The association of PE and CTEPH can lead to mortality of 70–90%12 but when correctly diagnosed, most patients can be treated by pulmonary endarterectomy.13 The pathophysiological continuum between the two entities appears to be influenced not only by incomplete resolution of the acute obstruction, leading to endothelialization of thrombi and thus occlusion or narrowing of pulmonary arteries, but also by histological changes in the pulmonary microvasculature.14 Various risk factors have been described that favor evolution of acute PE to CTEPH, including age (both younger10 and older15), previous PE,10 larger perfusion defect,10 idiopathic presentation,9,10 pulmonary artery systolic pressure (PASP) at admission ≥50 mmHg,11,16 and initial thrombus size.13 However, patient selection criteria in these studies were not specific in terms of PE risk stratification at admission.7–11
The aim of this study was to determine risk factors for the development of pulmonary hypertension (PH) in a population of patients with acute intermediate-to-high risk PE.
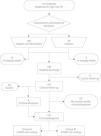
MethodsData collectionA retrospective analysis was performed of a prospective database of 213 consecutive patients admitted to the coronary care unit of Coimbra University Hospitals with a diagnosis of intermediate-to-high risk PE, between May 13, 2000 and October 15, 2010. The diagnosis was confirmed by computed tomography (CT) angiography or ventilation/perfusion scintigraphy. Clinical data collected at admission enabled retrospective risk stratification based on current guidelines6; this was complemented by analysis of patients’ medical records. The decision to perform fibrinolysis with alteplase (Actylise®, Boehringer Ingelheim, Germany) was at the discretion of the attending physician, and most patients (n=183) received fibrinolytic therapy. Patients were initially anticoagulated with unfractionated heparin, together with warfarin. Inotropic, vasopressor and ventilatory support were instituted depending on clinical assessment.
The following risk factors for PE were analyzed: advanced age (≥80 years), obesity, lower limb varicose veins, recent immobilization, known thrombophilia, previous venous thromboembolism, pregnancy, use of oral contraceptives, known cancer, heart failure, and recent major trauma or major surgery. Other demographic, clinical, laboratory and imaging variables were also recorded. Patients with a body mass index (BMI) of ≥30 kg/m2 were considered obese. Median follow-up was three years for 187 (95.4%) of the 196 patients who were discharged from hospital.
Transthoracic echocardiographyPatients with PASP of ≥40 mmHg were considered to have PH, as estimated by transthoracic echocardiography through calculation of the pressure gradient of tricuspid regurgitation using the formula PASP=4(VTR)2+central venous pressure. Central venous pressure was estimated by analysis of the respiratory variation of the inferior vena cava.17 Median time to echocardiography was 22 months (interquartile range 13–38, maximum 137). Only echocardiograms performed in the same hospital at least three months after discharge were considered valid; of the 187 patients, 121 (56.8% of the total) met this requirement. For individuals who underwent serial echocardiography during follow-up, only the most recent exam was used in the analysis. Patients were divided into two groups: group A (PASP <40 mmHg) with 106 patients (87.6%), and group B (PASP ≥40 mmHg) with 15 patients (12.4%).
Statistical analysisContinuous variables with normal distribution were expressed as means±standard deviation, while those showing an abnormal distribution were assessed by the Kolmogorov-Smirnov test and expressed as medians and interquartile range. Categorical variables were presented as absolute frequencies and percentages. Comparisons between groups were made using the Student's t test, Mann–Whitney U test or chi-square test, depending on the type of variable. In all tests, a p value of <0.05 was considered statistically significant. Parameters showing a significant association in univariate analysis with the development of PH during follow-up were included in a multivariate model. The best cut-off for each of these variables was determined by receiver-operator characteristic (ROC) curves. SPSS version 20.0 (Chicago, Illinois, USA) was used for the statistical analysis.
ResultsDemographic and clinical characteristicsOf the 213 patients included in the study, 61.5% were women, and mean age was 61.1±18.1 years. Most (90.6%) presented at least one risk factor for PE, while 20 cases (9.4%) were considered idiopathic; there was no significant difference in this respect between those with or without PH after PE (13.2 vs. 6.7%, p=0.692) (Figure 1).
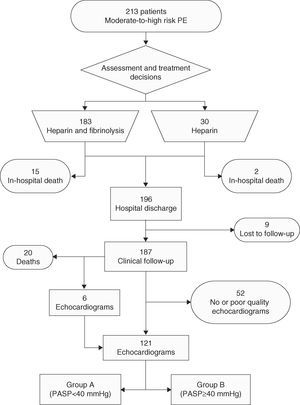
There were significant differences in PE risk factors (Table 1) between the two groups in age and use of oral contraceptives. Less than 3% of group A were aged >79 years at the time of PE diagnosis, as opposed to 53.3% of group B, and group B patients were older than group A (70.7±11.8 vs. 54.7±17.3, p<0.001) at diagnosis (Figure 2). No patients in group B were taking oral contraceptives at the time of admission, while around a quarter of those in group A were. The prevalence of other risk factors was similar in the two groups. Group B had a higher proportion of men (60.0 vs. 31.1%, p=0.041).
Frequency of risk factors for pulmonary embolism in the two study groups.
| Variable | Group A (n=106) | Group B (n=15) | p |
| Age ≥80 years, n | 3/106 (2.83%) | 8/15 (53.33%) | 0.002 |
| Thrombophilia, n | 2/106 (1.89%) | 0/15 (0%) | 1.000 |
| Lower limb varicose veins, n | 27/106 (27.36%) | 3/15 (20.00%) | 0.760 |
| Previous venous thromboembolism, n | 29/106 (27.36%) | 2/15 (13.33%) | 0.350 |
| Pregnancy, n | 1/106 (0.94%) | 0/15 (0%) | 1.000 |
| Oral contraceptives, n | 27/106 (25.47%) | 0/15 (0%) | 0.022 |
| Cancer, n | 11/106 (10.38%) | 0/15 (0%) | 0.356 |
| Heart failure, n | 8/106 (7.55%) | 0/15 (0%) | 0.594 |
| Major trauma, n | 12/106 (11.32%) | 1/15 (6.66%) | 1.000 |
| Major surgery, n | 20/106 (18.87%) | 1/15 (6.66%) | 1.000 |
| Idiopathic, n | 14/106 (13.21%) | 1/15 (6.66%) | 0.691 |
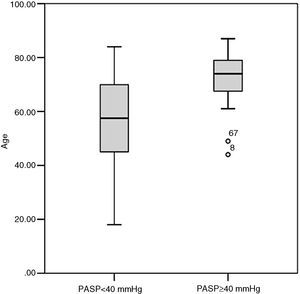
Group B had higher BMI than group A (31.3 [27.3–37.8] kg/m2 vs. 27.6 [25.0–30.8] kg/m2, p=0.036) and thus a higher prevalence of obesity (66.67% vs. 29.63%, p=0.023) (Figure 2). At admission, group A patients had significantly lower systolic blood pressure than group B (113 [110–140] mmHg vs. 140 [120–152] mmHg, p=0.027) (Table 2). A minority (5.6%) of patients presented hypotension at admission, and were diagnosed with high-risk PE; of these, 50% died before follow-up echocardiography (three during hospital stay and three after discharge). None of the six survivors of high-risk PE developed PH during follow-up (Figure 3).
Patient characteristics at admission.
| Variable | Group A (n=106) | Group B (n=15) | p |
| Age | |||
| Mean, years | 54.66±17.29 | 70.73±11.76 | 0.000 |
| ≥68 years, n | 29/106 (27.36%) | 11/15 (73.33%) | 0.001 |
| Gender | |||
| Male, n | 33/106 (31.13%) | 9/15 (60%) | 0.041 |
| Female, n | 73/106 (68.87%) | 6/15 (40%) | |
| BMI | |||
| Mean, kg/m2 | 27.55 (24.97–30.81) | 31.25 (27.27–37.72) | 0.036 |
| ≥30 kg/m2, n | 24/81 (29.63%) | 8/12 (66.67%) | 0.023 |
| HR, bpm | 94.00 (80.00–104.50) | 97 (85.00–101.50) | 0.714 |
| Systolic BP | |||
| Mean, mmHg | 122.50 (110.00–140.00) | 140.00 (120.00–152.00) | 0.027 |
| <90 mmHg, n | 6/106 (5.66%) | 0/15 (0.00%) | 0.594 |
| ≥135 mmHg, n | 32/106 (30.19%) | 10/15 (66.67%) | 0.009 |
| History | |||
| PE, n | 5/106 (4.72%) | 0/15 (0.00%) | 1.000 |
| HTN, n | 49/106 (46.23%) | 11/15 (73.33%) | 0.058 |
BMI: body mass index; BP: blood pressure; HR: heart rate; HTN: hypertension; PE: pulmonary embolism.
Analysis of arterial blood gas data showed no significant differences between the two groups, although group B had higher hemoglobin than group A (15.1 vs. 13.3 g/dl, p=0.014) (Table 3). There were no significant differences between the groups in imaging findings at admission or in therapeutic approach, including use of fibrinolysis. Recurrence of PE during follow-up was similar in the two groups, seven cases (6.6%) in group A and one (6.7%) in group B.
Diagnostic exams at admission.
| Variable | Group A (n=106) | Group B (n=15) | p |
| Arterial blood gas analysis | |||
| O2 saturation, % | 93.70 (90.18–95.40) | 92.00 (88.93–94.15) | 0.275 |
| pO2, mmHg | 62.70 (52.88–71.33) | 61.15 (53.40–67.43) | 0.722 |
| pCO2, mmHg | 31.50 (28.10–35.00) | 32.55 (28.00–37.68) | 0.483 |
| HCO3− mEq/l | 21.95 (19.95–23.75) | 22.20 (19.53–23.35) | 0.986 |
| Venous blood | |||
| Hemoglobin, g/dl | 13.30 (12.20–14.48) | 15.10 (12.20–16.00) | 0.014 |
| Platelets, 109/l | 225.00 (174.00–275.00) | 190.00 (171.00–224.00) | 0.079 |
| Troponin I, ng/ml | 0.15 (0.05–0.46) | 0.36 (0.04–1.75) | 0.538 |
| D-dimers, μg/ml | 5.35 (3.50–10.03) | 8.00 (5.60–23.10) | 0.073 |
| Fibrinogen, mg/dl | 3.75 (3.00–4.65) | 3.40 (2.85–4.29) | 0.649 |
| INR | 1.17 (1.07–1.30) | 1.25 (1.10–1.42) | 0.224 |
| aPTT, s | 27.30 (24.33–32.78) | 30.60 (26.90–33.00) | 0.153 |
| Echocardiography | |||
| Mean PASP, mmHg | 51.01±14.49 | 56.64±23.30 | 0.453 |
| PASP ≥40 mmHg, n | 58/74 (78.38%) | 9/11 (81.82%) | 1.000 |
| PASP ≥50 mmHg, n | 34/74 (45.95%) | 5/11 (45.45%) | 1.000 |
| CT angiography | |||
| Thrombi in main branches, n | 66/78 (84.62%) | 7/10 (70.00%) | 0.365 |
aPTT: activated partial thromboplastin time; HCO3−: bicarbonate level; INR: international normalized ratio; PASP: pulmonary artery systolic pressure; pCO2: partial carbon dioxide pressure; pO2: partial oxygen pressure.
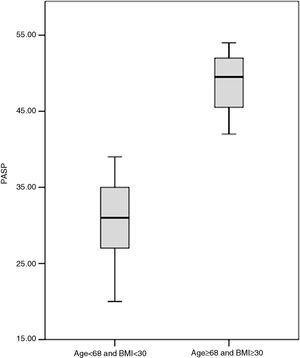
The following parameters were included in the multivariate analysis: age (p=0.001), gender (p=0.041), BMI (p=0.036), systolic blood pressure (p=0.027), hemoglobin (p=0.014), and use of oral contraceptives (p=0.022). Age and BMI were predictors of the development of PH, with hazard ratio of 1.09 (95% confidence interval [CI] 1.02–1.20, p=0.012) per year of age, and 1.19 (95% CI 1.04–1.36, p=0.013) per kg/m2 of BMI. Figure 4 shows the significant difference (p<0.001) in PASP between patients aged <68 years and BMI <30 kg/m2 (31.2±4.6 mmHg) and those aged ≥68 years and BMI ≥30 kg/m2 (48.8±5.0 mmHg).
DiscussionIn this study we identified risk factors for the development of PH after intermediate-to-high risk PE in a cohort of 213 patients, most of whom underwent fibrinolysis. Of the 121 patients who underwent echocardiography, 12.4% had PH, advanced age and obesity being identified as risk factors for its development.
The incidence of PH in our series was higher than in other studies. In 2004, Pengo et al. reported a significantly lower incidence (1.0% at six months and 3.8% at two years)10; however, as in the studies by Kline et al. (7.0%)7 and Klok et al. (0.6%),8 no data were presented on mortality risk stratification of PE at admission. The same applies to the study by Becattini et al.,9 who reported a low incidence of PH after PE (0.8% at three years). This may be due to the fact that echocardiography was only performed in patients presenting persistent dyspnea during follow-up. However, patients with PH can be asymptomatic for months or years, only presenting with the disease at a late stage.18 Since accentuation of the pulmonary component of the second heart sound may be the only sign, selecting only symptomatic patients for echocardiography may underestimate its real incidence.
In our population, age was shown to be a predictor of development of PH after PE, in agreement with the findings of Ribeiro et al.,11 who found that age >70 years was a risk factor for CTEPH, although paradoxically, Pengo et al.,10 reported a higher incidence of CTEPH in patients who were younger at the time of PE diagnosis. The greater incidence of PH in older patients may also be due to the fact that recurrent thromboembolic phenomena are more common in the elderly.12 It should be noted that as most studies were based on echocardiography, other forms of PH that cause elevated pulmonary pressure cannot be excluded, such as left ventricular diastolic dysfunction.19 Further studies will be required to determine the true impact of age as a risk factor for progression to CTEPH.
Obesity was also identified as a risk factor for the development of PH after PE, with each one-unit increment in BMI increasing the likelihood of PH by around 20%. This finding is particularly interesting, since it has not previously been described in the literature. There are various reasons that could explain this association. Dyslipidemia, strongly linked to obesity,20 may cause remodeling of the pulmonary microvasculature downstream of the obstruction, which is associated not only with CTEPH but also with other causes of PH.14 Each one-unit increment in BMI has been shown to raise PASP by 0.1–0.4 mmHg.21 Obesity may also contribute to development of PH in other ways, including increased cardiac output22 and association with other well-known causes of PH, particularly obstructive sleep apnea and right heart failure.17
In our population, a previous history of PE, idiopathic PE and a large perfusion defect, all described as risk factors for CTEPH,10 did not present statistically significant differences between the two groups (assuming that thrombus in a main branch indicates an extensive anatomical obstruction). Similarly, no correlation was found between initial thrombus size and CTHPH,13 nor was there any significant difference in PASP at admission, unlike the findings of other authors.11 However, the absence of these associations should be interpreted in the light of the clinical severity of most patients at the time of diagnosis, reflecting the presence of a sufficiently large thrombus to obstruct a major branch of the pulmonary vascular tree and to cause a large perfusion defect. This led to greater use of fibrinolysis (86% in our study compared to 7–41% in other series in the literature7,9-11) (Table 4), which resolves the obstruction more rapidly than treatment with heparin only.6 Furthermore, there are preliminary data suggesting that fibrinolysis may reduce the incidence of CTEPH.23
CTEPH is the only curable form of pulmonary hypertension. Early and accurate diagnosis is essential as this enables pulmonary endarterectomy to be performed, which, although a complex surgical technique, leads to total recovery of functional capacity with mortality of less than 5% in experienced centers.17,23 For the minority of patients with inoperable CTEPH, pulmonary vasodilators may be indicated.17 A recent multicenter clinical trial (CHEST-1) showed that riociguat, a soluble guanylate cyclase stimulator, improves functional capacity in patients with inoperable CTEPH as assessed by the 6-minute walk test.24
Study limitationsThe retrospective nature of the present study means it has various limitations, of which the main one is the fact that a diagnosis of PH was based on echocardiographic assessment rather than direct catheterization, the gold-standard method.6 Thus, besides the possibility of some patients being false positives or false negatives, it was not possible to identify concomitant causes of PH such as left ventricular dysfunction. We would stress that only direct catheterization or pulmonary angiography (or at least ventilation/perfusion scintigraphy) can confirm a diagnosis of CTEPH, since CT angiography is associated with a false negative rate of 50%.25 Accordingly, we prefer to use the term PH after PE, since a diagnosis of CTEPH requires use of one of the diagnostic techniques mentioned above.
A further limitation is the lack of echocardiographic follow-up in part of the study population, which may have introduced bias in assessment of PH prevalence. Only use of a prospective protocol can mitigate this important limitation. Nevertheless, we feel that this first analysis of the incidence of PH after intermediate-to-high risk PE may call attention to the problem and that an incidence of around 10% at three years may prompt closer clinical follow-up of these patients, particularly among the elderly and/or obese. Naturally, differential diagnosis with other causes of PH remains crucial for the appropriate selection of patients for surgical or medical interventions.
ConclusionsIn our population, the incidence of PH after intermediate-to-high risk PE was 12%, and we identified advanced age and obesity as predisposing factors. Our results suggest that older and/or obese patients should undergo close clinical and echocardiographic monitoring after PE, as this will help prompt detection of PH and enable intervention in the earlier phases of the disease.
Conflicts of interestThe authors have no conflicts of interest to declare.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Please cite this article as: Barros A, Baptista R, Nogueira A, et al. Preditores de hipertensão pulmonar após tromboembolia pulmonar de risco intermédio a elevado. 2013;32:857–864.