Reducing low-density lipoprotein cholesterol (LDL-C) levels is one of the most important strategies for reducing the risk of cardiovascular events. However, in clinical practice, a high proportion of patients do not achieve recommended LDL-C levels through lifestyle and lipid-lowering therapy with statins and ezetimibe. PCSK9 inhibitors (PCSK9i) are a new therapeutic option that significantly (50-60%) reduces LDL-C levels, which in clinical trials translates into an additional reduction in risk for cardiovascular events, and has a good safety profile. However, this therapy is associated with significant costs, and therefore its use in clinical practice should take its cost-effectiveness into account. Priority should be given to use in patients at higher cardiovascular risk and those in whom high LDL-C levels persist despite optimal lipid-lowering therapy.
This consensus document aims to summarize the main data on the clinical use of PCSK9i and to make recommendations for Portugal on the profile of patients who may benefit most from this therapy.
A redução dos valores de c-LDL é uma das estratégias mais importantes para a redução do risco de eventos cardiovasculares. Contudo, na prática clínica, um grande número de doentes não atinge os valores recomendados de c-LDL com a modificação do estilo de vida e a terapêutica hipolipemiante com estatinas e ezetimiba.
Os fármacos inibidores da PCSK9 (iPCSK9) são uma nova opção terapêutica que permite uma redução significativa dos valores de c-LDL (50-60%), que se traduziu nos ensaios clínicos numa redução adicional do risco de eventos cardiovasculares, com bom perfil de segurança. Porém, é uma terapêutica com custos elevados, pelo que a utilização na prática clínica deve ter em conta a sua custo-efetividade devendo ser dada prioridade à utilização nos doentes de maior risco cardiovascular e que mantêm valores elevados de c-LDL apesar da terapêutica hipolipemiante otimizada.
Este documento de consenso, tem como objetivo resumir os principais dados sobre a utilização clínica dos iPCSK9 e estabelecer recomendações para Portugal sobre o perfil de doentes que mais pode beneficiar desta terapêutica.
Cardiovascular disease (CVD) is the leading cause of death in Portugal1 and in other developed countries. Various risk factors for atherosclerotic (atherothrombotic) CVD are known, one of the most important of which is dyslipidemia. In recent years, findings from epidemiological, genetic, pathophysiological and clinical studies have shown unequivocally that there is a clear, consistent and progressive association between higher plasma low-density lipoprotein (LDL) cholesterol (LDL-C) levels and atherosclerotic CVD, confirming that LDL-C is a direct causal factor of the disease.2
Furthermore, there is incontrovertible evidence that reducing LDL-C proportionally reduces the risk of cardiovascular (CV) events.3,4 The guidelines therefore consistently recommend that lipid-lowering therapy should be adjusted in accordance with target LDL-C levels, which should be individualized on the basis of the patient's CV risk.5–7
However, various studies have shown that in a high proportion of patients, CV risk factors are not adequately controlled.8 The Portuguese results of the Dyslipidemia International Study (DYSIS) showed that 63% of patients treated with statins did not reach target LDL-C levels.9 This poor LDL-C control is due to many factors, which may be dependent on the patient (poor adherence and discontinuation of therapy), on the physician (particularly clinical inertia), or on the treatment (interindividual variations in and limits to the efficacy of lipid-lowering therapy).5,10
In recent years, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors (PCSK9i) have emerged as a new therapeutic option that can consistently achieve significant (∼50-60%) reductions in LDL-C levels, which in clinical trials translates into an additional reduction in risk for CV events.11–13 However, PCSK9i are a costly therapy and should therefore be used only after careful consideration of their cost-effectiveness and their impact on the sustainability of the health system.14
This consensus document, produced by specialists in cardiology, endocrinology and internal medicine and sponsored by the corresponding medical societies, aims to summarize the main data on the clinical use of PCSK9i and to make recommendations suitable for the situation in Portugal on the profile of patients who may benefit most from this therapy.
The ‘LDL hypothesis’: the lower the better?The association between LDL-C levels and the development of atherosclerosis (the ‘LDL hypothesis’) was established decades ago, based on numerous epidemiological studies demonstrating a continuous and linear relationship between exposure to high plasma LDL-C levels and risk of CV events.15
More recent data from genetic studies, particularly those using Mendelian randomization, have consistently shown that multiple genetic variants that lead to higher LDL-C levels are associated with increased CV risk,16,17 and confirm that LDL-C levels play a causal role in the development of CVD.2
The most significant evidence on the role of LDL-C as a cause of CVD comes from randomized clinical trials that demonstrate a proportional relationship between the extent of LDL-C reduction and reduction of CV risk. A meta-analysis of 26 trials involving 17 000 individuals on statin therapy revealed a 22% reduction in risk of major CV events per 1.0 mmol/l (39 mg/dl) reduction in LDL-C, establishing the concept of ‘the lower, the better’.3,18,19 The benefit of lipid-lowering therapy is less in the first year of treatment, when the risk reduction is ∼10%, but is consistently 22-24% per mmol/l in subsequent years.20,21 The risk reduction is seen with different therapeutic options (statins, ezetimibe, resins, PCSK9i or ileal bypass surgery) that have in common the effect of reducing LDL-C by upregulating LDL receptor (LDLR) expression in hepatocytes, as demonstrated by a meta-analysis of 49 trials including 312 175 patients and 39 645 events.4 Finally, clinical trials assessing progression of coronary disease by intravascular ultrasound have shown that progression of atherosclerotic plaque can be halted when LDL-C levels are reduced to below 70 mg/dl.22,23
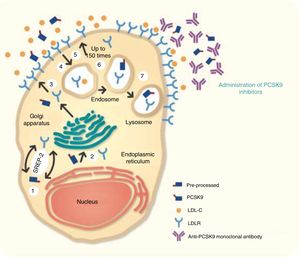
PCSK9 inhibition: from mechanism of action to cardiovascular outcomes trialsPCSK9 is a serine protease that reduces LDLR levels. Inhibition of PCSK9 thus increases the number of LDLRs on the surface of hepatocytes. PCSK9i are monoclonal antibodies (mAbs) that bind to PCSK9, preventing it from binding to LDLRs, thereby reducing serum LDL-C levels (Figure 1).24,25
Mechanism of action of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. In the hepatocyte, sterol regulatory element-binding protein 2 (SREBP-2) regulates the transcription of various lipids and proteins, including the low-density lipoprotein (LDL) receptor (LDLR) and PCSK9. In step 1, PCSK9 is processed in the endoplasmic reticulum (step 2), from where the mature form passes to the Golgi apparatus before being secreted (step 3). On the hepatocyte surface, the LDL receptor (LDLR) binds to circulating LDL cholesterol (LDL-C), and the resulting complex is internalized to the endosome (step 4). The LDLR then recirculates to the cell surface (step 5). This process occurs up to 150 times, increasing the quantity of LDL-C removed from the circulation. PCSK9 regulates the quantity of LDLRs on the hepatocyte surface by binding to the receptor, which is then internalized and degraded in the lysosome (steps 6 and 7), preventing LDLRs from being recycled to the cell surface.
The gene for PCSK9 was identified around 15 years ago in the context of research into the phenotype of familial hypercholesterolemia (FH), which was soon shown to be associated with a gain-of-function mutation in this gene.26,27 The subsequent discovery of loss-of-function PCSK9 variants associated with lower serum LDL-C levels and reduced CV risk led to the development of the pharmacological model underlying PCSK9i.28 Anti-PCSK9 mAbs were soon developed and a series of clinical research programs were established to test the ability of PCSK9i to reduce CV events.29 At the same time, efforts continued to develop alternate ways of inhibiting PCSK9 (Table 1).30,31
Different forms of PCSK9 inhibition.
| Technology | Agent | Clinical research program | Status |
|---|---|---|---|
| Anti-PCSK9 monoclonal antibody | Alirocumab | ODYSSEY | EMA approval |
| Bococizumab | SPIRE | Withdrawn | |
| Evolocumab | PROFICIO | EMA approval | |
| PCSK9 gene silencing | |||
| Small interfering RNA | Inclisiran | ORION | Phase III |
| Antisense oligonucleotides | BMS-84442, SPC5001, SPC4061 | Phase I | Withdrawn |
| Small-molecule inhibitors of PCSK9 | |||
| Adnectin | BMS-962476 | - | Phase I |
| Annexin | Annexin A2 | - | Pre-clinical |
| Vaccine | AT04A | - | Pre-clinical |
EMA: European Medicines Agency.
Three mAbs have been developed as PCSK9i: alirocumab, bococizumab and evolocumab. They act by binding to PCSK9, preventing it from binding to LDLRs and being degraded in the lysosome, and thereby increasing the number of LDLRs (Figure 1).25 Alirocumab and evolocumab are fully human immunoglobulins (Ig) of the IgG1 and IgG2 isotypes, respectively, while bococizumab is a humanized IgG2. All are administered subcutaneously, and since they are complete antibodies their half-life is long (one injection every 2-4 weeks).
Effect on lipid profileAfter administration of 140 or 420 mg of evolocumab, mean peak serum concentration is reached in 3-4 days. Its absolute bioavailability is 72% and its effective half-life is 11-17 days. Repeated administration leads to dose-proportional increases in exposure, with trough serum concentrations approaching steady state by 12 weeks of dosing. A single administration of evolocumab results in early maximum suppression (almost complete) of circulating unbound PCSK9 by four hours, followed by a reduction in LDL-C reaching a mean nadir by 14-21 days. Changes in unbound PCSK9 and serum lipoproteins are reversible on discontinuation of the drug, without evidence of rebound.
The pharmacokinetics of alirocumab is similar. Mean time to peak serum concentration after administration is 3-7 days, absolute bioavailability is about 85% and steady state is reached after 2-3 doses. Median apparent half-life at steady state is 17-20 days.32,33
Anti-PCSK9 mAbs reduce LDL-C levels by around 60%, as shown in various short-term studies (Table 2),34,35 independently of first-line lipid-lowering therapy.36 Their effect is slightly greater in individuals taking statins, since the latter increase expression of the PCSK9 gene.37 As well as reducing LDL-C levels, anti-PCSK9 mAbs also reduce total cholesterol by about 38%, apolipoprotein B (ApoB) by 50% and lipoprotein(a) (Lp(a)) by 28%. They reduced LDL-C levels by 51.4% in statin-intolerant patients.38–41
Percentage difference in lipid parameters between anti-PCSK9 monoclonal antibodies and placebo at 12/24 weeks.34,35
| TC | LDL-C | HDL-C | TG | Lp(a) | ApoB | |
|---|---|---|---|---|---|---|
| Alirocumab | -39.0 | -60.4 | +6.3 | -9.3 | -26.8 | -50.2 |
| Bococizumab | -34.1 | -57.1 | +6.0 | -14.2 | -24.2 | -46.0 |
| Evolocumab | -42.0 | -71.4 | +12.8 | -17.4 | -45.0 | -56.4 |
ApoB: apolipoprotein B; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; Lp(a): lipoprotein(a); TC: total cholesterol; TG: triglycerides.
Long-term LDL-C reduction was maintained with alirocumab (-54.7% at 48 months) and evolocumab (-54.0% at 39 months) in cardiovascular outcomes trials.11,13 However, a reduction in efficacy was seen with bococizumab, a humanized anti-PCSK9 mAb (-38.3% at 24 months).12 This is usually attributed to the development of neutralizing antidrug antibodies against bococizumab, which occurred in around a third of patients within a year. As s result, the clinical development program of this drug has been discontinued.35
In terms of pharmacodynamics, anti-PCSK9 mAbs reduce LDL-C in all patients who can expose hepatic LDLRs. In trials of patients with heterozygous FH (HeFH) under statin therapy with or without ezetimibe, anti-PCSK9 mAbs significantly reduced LDL-C levels, by 39.1-61.3%, irrespective of the type of LDLR mutation.42 In homozygous FH (HoFH), reductions in LDL-C with evolocumab were highly variable, with percentage falls ranging between 22.9% and 40.8% in patients with a deleterious LDLR mutation in at least one allele.42–44 Patients with HoFH, having a doubly defective LDLR gene, are naturally much less likely to respond to lipid-lowering treatment than those with HeFH. Rare cases have been described of absent or weak response to PCSK9i. Response is variable in autosomal recessive hypercholesterolemia (caused by mutations in the LDLRAP1 gene that codes for low-density lipoprotein receptor adapter protein 1, which is involved in internalization of the LDLR/LDL complex in hepatocytes, leading to increased LDL-C levels) and in cases of the R410S mutation in the LDLR gene, which also alters LDLR metabolism in the endosome. Genetic evaluation is therefore indicated in patients who fail to respond to PCSK9i.
It should also be noted that these drugs do not reduce C-reactive protein levels, suggesting that statins’ effect on this biomarker may be a pleiotropic effect and independent of LDL-C reduction.45,46
Cardiovascular outcomes trialsThe anti-PCSK9 mAbs have been assessed in four randomized placebo-controlled cardiovascular outcomes trials that included more than 70 000 patients, most in the context of secondary prevention (Table 3).11–13
Cardiovascular outcomes trials on anti-PCSK9 monoclonal antibodies.
| ODYSSEY OUTCOMES13 | SPIRE-112 | SPIRE-212 | FOURIER11 | |
|---|---|---|---|---|
| Agent | Alirocumab | Bococizumab | Evolocumab | |
| Dose | 75 or 150 mg every 2 weeks (adjusted to target LDL-C level of 25-50 mg/dl) | 150 mg every 2 weeks (reduced to 75 mg every 2 weeks if LDL-C <10 mg/dl) | 140 mg every 2 weeks or 420 mg every 4 weeks | |
| No. of patients included | 18 924 | 16 817 | 10 621 | 27 564 |
| Population | ACS | Secondary (84%) or primary prevention with high CV risk (16%) | MI (81%), stroke (19%) or PAD (13%) | |
| Mean age, years | 58.6 | 63.3 | 62.4 | 62.5 |
| Diabetes (%) | 29 | 48 | 47 | 37 |
| Eligibility (lipid levels), mg/dl | LDL-C ≥70 or non-HDL-C ≥100 or ApoB ≥80 | LDL-C ≥70 or non-HDL-C ≥100 | LDL-C ≥100 or non-HDL-C ≥130 | LDL-C ≥70 or non-HDL-C ≥100 |
| Baseline LDL-C, mg/dl | 92 | 94 | 134 | 92 |
| LDL-C level attained (mg/dl) | 53 (vs. 101 placebo) | 44 (vs. 100 placebo) | 80 (vs. 137 placebo) | 30 (vs. 86 placebo) |
| Intensive statin therapy, % | 89 | 92 | 73 | 69 |
| Duration of trial, years | 2.8 | 0.6 | 1 | 2.2 |
| Primary endpoint, HR (95% CI) | 0.85 (0.78-0.93)a; NNT: 63 | 0.99 (0.80-1.22)b | 0.79 (0.65-0.97)b | 0.85 (0.79-0.92)c; NNT: 67 |
| CV death, MI or stroke, HR (95% CI) | 0.86 (0.79-0.93)d; NNT: 63 | 1.03 (0.82-1.30) | 0.74 (0.60-0.92) | 0.80 (0.73-0.88); NNT: 67 |
| CV death, HR (95% CI) | 0.88 (0.74-1.05) | 1.20 (0.74-1.95) | 0.82 (0.50-1.36) | 1.05 (0.88-1.25) |
| MI, HR (95% CI) | 0.86 (0.77-0.96)e; NNT: 100 | 1.11 (0.83-1.48)e | 0.76 (0.58-1.00)e | 0.73 (0.65-0.82); NNT: 84 |
| Stroke, HR (95% CI) | 0.73 (0.57-0.93); NNT: 250 | 0.52 (0.30-0.91)e | 0.66 (0.40-1.09)e | 0.79 (0.66-0.95); NNT: 250 |
ACS: acute coronary syndrome; ApoB: apolipoprotein B; CI: confidence interval; CV: cardiovascular; HR: hazard ratio; LDL-C: low-density lipoprotein cholesterol; MI: myocardial infarction; NNT: number needed to treat; non-HDL-C: non-high-density lipoprotein cholesterol; PAD: peripheral arterial disease; UA: unstable angina.
The FOURIER trial, which tested evolocumab for secondary prevention in 27 564 patients with stable established cardiovascular disease, was the first to demonstrate significant reductions in CV events with PCSK9i (Table 3).11 Evolocumab reduced the relative risk of the primary combined endpoint of CV death, myocardial infarction (MI), stroke, hospitalization for unstable angina (UA) or coronary revascularization by 15%. Significant reductions were seen in the relative risk of MI (27%), stroke (21%), and revascularization (22%), but not of CV death or UA. The results were consistent irrespective of baseline LDL-C levels or standard lipid-lowering therapy. The effect of evolocumab on CV events was more pronounced beyond the first year, with relative risk reductions of 19% in the primary endpoint and 25% in the secondary endpoint compared to placebo, as has also been observed in trials of statins.18 The magnitude of the reduction in relative risk for CV events with evolocumab per mmol/l reduction in LDL-C was comparable to that in the Cholesterol Treatment Trialists Collaboration.3 Analysis of the absolute risk reduction in the primary and key secondary endpoints showed greater benefit for patients at higher risk, with a lower number needed to treat (NNT) in patients with multivessel disease, multiple prior MIs or MI in the previous two years (NNT 34 and 40 for the primary and key secondary endpoints, respectively), peripheral arterial disease (NNT 29 for both endpoints), and TIMI risk score >4 (NNT 28) than in the overall population (NNT 67 for both endpoints).36,47,48
The SPIRE-1 and SPIRE-2 trials tested the effect of bococizumab in a total of 27 438 patients, most for secondary prevention. The inclusion criteria in SPIRE-2 had higher thresholds for LDL-C and non-high-density lipoprotein cholesterol than in SPIRE-1 (Table 3).12 The two trials were halted early because attenuation in the reduction in LDL-C levels was observed over time. Although bococizumab reduced the relative risk of the primary endpoint of CV death, MI, stroke or UA requiring urgent revascularization by 12% and the secondary composite endpoint of CV death, MI or stroke by 13%, these differences were not statistically significant. In SPIRE-2, in which exposure to bococizumab was longer and baseline LDL-C was higher, statistically significant reductions were seen in the primary (21%) and secondary (26%) endpoints compared to placebo.
Finally, the ODYSSEY OUTCOMES trial on alirocumab included 18 924 patients with recent acute coronary syndrome (ACS) under statin therapy, with a median follow-up of 2.8 years (Table 3).13 Alirocumab significantly reduced the relative risk of the primary endpoint of coronary heart disease, non-fatal MI, fatal or non-fatal ischemic stroke, or UA requiring hospitalization by 15%, and of the secondary endpoint of death from any cause, non-fatal MI or non-fatal stroke by 14%. There were also significant reductions in non-fatal MI (14%), stroke (27%), UA (39%) and revascularization (12%). However, alirocumab did not significantly reduce coronary or CV death. The benefit was consistent in all subgroup analyses. In a post hoc analysis, a significant reduction of 28% was seen in the relative risk of coronary death (28%) and of CV death (31%) compared to placebo.
In summary, cardiovascular outcomes trials on anti-PCSK9 mAbs, taken together, support the concept of a linear association between LDL-C levels achieved and the rate of CV events, irrespective of the LDL-C levels achieved.
Use of PCSK9 inhibitors in familial hypercholesterolemiaFH is associated with increased risk of premature CVD.49 Individuals with LDL-C levels >190 mg/dl are considered to be at high CV risk,5 and most such individuals have FH, either monogenic (caused by mutations in genes involved in LDL-C metabolism) or polygenic (resulting from interactions between an atherogenic diet and a variety of genetic factors, some not yet identified).
The population with FH is heterogeneous, due to the large number of causative mutations. The degree of atherosclerotic disease and of CV risk depend mainly on LDL-C levels and length of exposure. The most common cause of monogenic FH is mutations in the LDLR gene (∼90%), followed by mutations in the APOBgene (∼5%), gain-of-function mutations in the PCSK9 gene (<1%), and very rarely loss-of-function mutations in the LDLRAP1 gene, which codes for a protein involved in internalization of LDLR/LDL-C complexes.50 HeFH has an estimated prevalence of 1/500-1/200 in the general population, while HoFH is much rarer (1/160 000-1/300 000).42 FH is underdiagnosed, particularly the heterozygous form, which is often only suspected after a premature coronary event (age <55 years in men and <60 years in women).
Lowering LDL-C levels reduces the risk of CV events in familial hypercholesterolemia, in which primary prevention is more effective than secondary prevention in reducing mortality from coronary disease (by 48% vs. 25%).51 Initiation of pharmacological treatment in FH is recommended as soon as possible, from 8-10 years of age, to reduce mortality in adulthood. There have been recent attempts to improve CV risk stratification in patients with FH in order to identify those at higher risk earlier and to start aggressive lipid-lowering therapy. The European guidelines on the management of dyslipidemias recommend a target of <100 mg/dl LDL-C for adults with FH.5
PCSK9i have been tested in various trials on FH. The PROFICIO program on evolocumab included patients with HeFH (RUTHERFORD-2) and HoFH (TESLA Part A and TESLA Part B), and the TAUSSIG trial is currently under way. The ODYSSEY program (alirocumab) only included patients with HeFH (Table 4).42
Trials assessing use of PCSK9 inhibitors in patients with familial hypercholesterolemia.
| Trial | Population | Reduction in LDL-C | Reduction in Lp(a) |
|---|---|---|---|
| Evolocumab | |||
| RUTHERFORD-252 | HeFH (12 weeks) | 140 mg every 2 weeks: -59.2%420 mg every 4 weeks: -61.3% | 140 mg every 2 weeks: -31.6%420 mg every 4 weeks: -28.2% |
| TESLA Part A53 | HoFH (36 weeks) | every 4 weeks: -16.5% every 2 weeks: -13.9% | every 4 weeks: -11.7% every 2 weeks: -18.6% |
| TESLA Part B44 | HoFH (12 weeks) | -30.9% | -11.8% (p=0.09) |
| TAUSSIG54 | Severe FH (5 years) | Under way | |
| Alirocumab | |||
| ODYSSEY FH I and FH II55 | HeFH (78 weeks) | FH I: -57.9% FH II: -51.4% | FH I: -17.7%FH II: -20.3% |
| ODYSSEY HIGH56 | HeFH(78 weeks) | -39.1% at week 24 | -14.8% |
| ODYSSEY LONG TERM57 | HeFH (78 weeks) | -61.9% at week 24 | -25.6% |
| ODYSSEY OLE58 | HeFH (176 weeks) | Under way | |
FH: familial hypercholesterolemia; HeFH: heterozygous familial hypercholesterolemia; HoFH: homozygous familial hypercholesterolemia; LDL-C: low-density lipoprotein cholesterol.
Adapted from Raal et al.42
These trials demonstrated the efficacy of PCSK9i, particularly in HeFH, achieving additional reductions of around 60% in patients under maximum lipid-lowering therapy and enabling 80% of these patients to achieve target LDL-C levels.
Other forms of PCSK9 inhibitionOligonucleotides, small single- or double-stranded DNA or RNA molecules consisting of 10-50 nucleotides that are synthetic analogs of natural nucleic acids, are an emerging area of interest for the treatment of CVD and lipid disorders. Therapies based on oligonucleotides are usually divided into two groups: one including antisense oligonucleotides, ribozymes, and small interfering RNA (siRNA), which suppress expression of a protein by hybridization to target messenger RNA (mRNA); and the other consisting of aptamers, which like antibodies bind to target proteins and inhibit their function.59
Inclisiran, a synthetic single-strand siRNA, is the alternative to mAbs that is at the most advanced stage of clinical development. It is conjugated to N-acetylgalactosamine in a formulation that is taken up by hepatocytes, where it cleaves PCSK9 mRNA, reducing its synthesis.60 The pharmacodynamic effect is prolonged, and so weekly parenteral administration is feasible. In the phase II ORION-1 trial, 300-mg subcutaneous doses at the beginning of the study and at three months reduced LDL-C by up to 52.6%.61
Safety of anti-PCSK9 monoclonal antibodiesIn general, anti-PCSK9 mAbs have been shown to be safe in cardiovascular outcomes trials (Table 5),11–13 confirming data from initial studies.36 Administration of these agents can result in local reactions such as erythema, swelling or itching, which are usually mild, the incidence of which ranges between 2.1% and 10.4% (Table 5). The highest incidence of such reactions was seen with bococizumab, which may be due to its partially murine origin.
Safety of anti-PCSK9 monoclonal antibodies in cardiovascular outcomes trials.
| Event | Odyssey outcomes13 | Spire-1 and Spire-212 | Fourier11 | |||
|---|---|---|---|---|---|---|
| Alirocumab | Placebo | Bococizumab | Placebo | Evolocumab | Placebo | |
| Adverse event | 75.8% | 77.1% | 63.7%a | 60.5% | 77.4% | 77.4% |
| Severe adverse event | 23.3% | 24.9% | 19.5% | 19.7% | 24.8% | 24.7% |
| Injection site reaction | 3.8%a | 2.1% | 10.4%a | 1.3% | 2.1%a | 1.6% |
| New-onset diabetes | 9.6% | 10.1% | 4.2% | 4.2% | 8.1% | 7.7% |
| Neurocognitive event | 1.5% | 1.8% | 1.6% | 1.5% | ||
| Elevated ALT (>3 times) | 2.3% | 2.4% | 0.8% | 0.9% | 1.8% | 1.8% |
| Elevated CKb | 0.5% | 0.5% | 1.0% | 0.9% | 0.7% | 0.7% |
| Neutralizing antidrug antibodies against PCSK9i | 0.5% | <0.1% | 29% | - | 0 | 0 |
No increase was seen in the incidence of new-onset diabetes (Table 5), contradicting the hypothesis that lower LDL-C levels could raise the risk of diabetes.62 This neutral effect on glycemic control was confirmed in a subanalysis of the FOURIER trial.63
No hepatic or muscular toxicity was observed. Neutralizing antidrug antibodies were detected in 43 patients (0.5%) in the alirocumab group in ODYSSEY OUTCOMES, but were not observed with evolocumab in FOURIER. As stated above, the frequent finding of neutralizing antidrug antibodies against bococizumab led to the discontinuation of its clinical development program. The most common adverse reactions to evolocumab were nasopharyngitis (4.8%), upper respiratory tract infection (3.2%), back pain (3.1%), arthralgia (2.2%), influenza (2.3%), and nausea (2.1%). The most common adverse reactions with alirocumab were local injection site reactions such as erythema, pain and hematoma.
Fears that very low LDL-C levels could be related to cognitive dysfunction have not so far been confirmed in clinical trials. In the EBBINGHAUS trial, which assessed neurocognitive function in detail in patients under evolocumab, no significant differences from placebo were seen at 24 weeks or at the end of the trial.64
Although the safety profile of anti-PCSK9 mAbs has been shown to be excellent in the medium term, the long-term effects of exposure to these agents are unknown. Genetic syndromes associated with FH, particularly those caused by loss-of-function mutations in the PCSK9 gene, may represent a model of prolonged exposure to PCSK9i.65 No neurocognitive disorders are reported in carriers of these mutations, except for one mutation in APOB (truncated isoform of ApoB29.4). However, the risk of hepatic steatosis is high (>70% in truncated isoforms of ApoB100).
Cost-effectiveness analyses of PCSK9 inhibitorsDespite the efficacy of PCSK9i in reducing CV events, they have been used relatively little in clinical practice,66 mainly due to their high price. Cost-effectiveness analyses of the therapy are therefore essential, and several such studies have been published. The results have been highly variable, with incremental cost-effectiveness ratios ranging between 30 000 and 166 000 euros per quality-adjusted life year67 depending on the simulation model, the profile of patients included in the analysis, the context of use, and the cost of the treatment.
A ‘highest risk-highest benefit’ strategy has been proposed to improve the cost-effectiveness of these agents.68 In such a strategy, these agents should be used in patients at the highest risk, i.e. those with the greatest likelihood of suffering a CV event, such as those with a history of CVD and polyvascular disease or diabetes. Furthermore, assuming that relative risk reduction in patients at high CV risk is proportional to the absolute reduction in LDL-C, and that the magnitude of percent LDL-C reduction with PCSK9i therapy is similar across baseline LDL-C subgroups, patients with the highest starting LDL-C will achieve the greatest absolute reduction in LDL-C and hence the greatest relative risk reduction (highest benefit). On the basis of this concept, these guidelines propose that PCSK9i should preferentially be used in patients who are most likely to benefit from them, taking into consideration the patient's CV risk and absolute LDL-C level.
With regard to the cost-effectiveness of PCSK9i in Portugal, although precise figures are lacking, in December 2018 the Portuguese National Authority for Medicines and Health Products (INFARMED) published its Public Assessment Report on in-hospital use of evolocumab, which stated that “the incremental cost-effectiveness analysis of the introduction of evolocumab into the therapeutic arsenal, and the resulting impact on budgets, were considered acceptable.”69
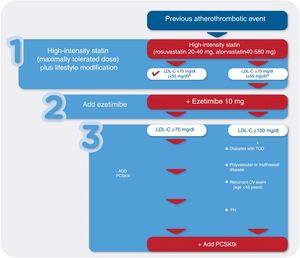
Recommendations for the use of PCSK9 inhibitors in secondary preventionPatients with established CVD, particularly those who have already suffered an atherothrombotic event, are at the highest risk for recurrent CV events.70 Given the association between LDL-C levels and risk of events, the therapeutic targets recommended in these patients are particularly strict, with the European guidelines recommending LDL-C levels <70 mg/dl,5 while recognizing that subgroups at extreme risk may benefit from even lower levels (<55 mg/dl).7
In these patients, the first-line therapy should be high-intensity statins (atorvastatin 40-80 mg or rosuvastatin 20-40 mg), together with dietary counseling and appropriate lifestyle changes. However, according to some published simulations, around 32% of patients will not attain target LDL-C levels solely with high-intensity statin therapy, and so other lipid-lowering agents will need to be added.71 The next step is to consider the addition of ezetimibe, a low-cost and well-tolerated drug, which will further reduce LDL-C levels,72 enabling a further 19% of patients to attain target levels. Even so, in around 14% of patients, LDL-C levels will remain above the target, and for these treatment with PCSK9i should be considered.
Rational use of these drugs entails identifying groups at the highest absolute risk and with the highest LDL-C levels, in whom the expected benefit is greatest. This is the approach recommended by most published guidelines on the use of PCSK9i (Table 6).
Summary of guidelines for the use of PCSK9 inhibitors in secondary prevention.
| Guidelines | Patient profile in secondary prevention | LDL-C threshold to consider introducing PCSK9ia |
|---|---|---|
| NICE73 | High risk: previous cardiovascular event (ACS, ischemic stroke, coronary or other arterial revascularization, coronary heart disease or PAD) | ≥150 mg/dl |
| Very high risk: polyvascular disease or recurrent CV events | ≥130 mg/dl | |
| SSC74 | ACS <1 year + familial hypercholesterolemia | >70 mg/dl |
| Stable coronary disease or previous ACS | >100 mg/dl | |
| Recurrent ACS | >70 mg/dl | |
| Coronary disease + statin intolerance or contraindication | Depending on target level | |
| ESC/ESA75 | Clinical CVD(ACS, PAD, previous stroke) | >140 mg/dl |
| Clinical CVD + one of the following:- Familial dyslipidemia- Diabetes with TOD or a major risk factor- Severe or extensive CVD (e.g. polyvascular)- Rapidly progressive CVD (recurrent ACS, repeated coronary revascularizations, or recurrent ischemic stroke, within 5 years of the index event) | >100 mg/dl | |
| ACC/AHA76 | Very high risk CVD | ≥70 mg/dlb |
Use in accordance with cost-effectiveness considerations.
ACC/AHA: American College of Cardiology/American Heart Association; ACS: acute coronary syndrome; CV: cardiovascular; CVD: cardiovascular disease; ESC/ESA: European Society of Cardiology/European Atherosclerosis Society; NICE: National Institute for Health and Care Excellence (UK); PAD: peripheral arterial disease; PCSK9i: PCSK9 inhibitors; SSC: Spanish Society of Cardiology; TOD: target organ damage.
In 2016, the UK National Institute for Health and Care Excellence (NICE) guidelines on the use of PCSK9i in patients with established CVD set LDL-C thresholds of 150 mg/dl for patients at high risk (ACS, ischemic stroke, coronary or other arterial revascularization, coronary heart disease or peripheral arterial disease) and 130 mg/dl for those at very high risk (polyvascular disease or recurrent CV events). The only case in which PCSK9i were to be considered for primary prevention was in HeFH with LDL-C >190 mg/dl.73 The Spanish Society of Cardiology recommends these drugs for secondary prevention in patients with ischemic heart disease and LDL-C >100 mg/dl despite optimal lipid-lowering therapy, and in those with LDL-C >70 mg/dl and recurrent ACS or ischemic heart disease and FH.74 They are also recommended in patients with ischemic heart disease and demonstrated statin intolerance, as well as those without known CVD but with LDL-C >130 mg/dl and type 1 or 2 diabetes with target organ damage, estimated glomerular filtration rate <60 ml/min/1.73 m2, or SCORE >10%. The European Society of Cardiology/European Atherosclerosis Society (ESC/ESA) guidelines recommend PCSK9i in patients at very high CV risk with clinical or imaging evidence of atherosclerotic disease who, despite recommended maximally tolerated statin plus ezetimibe therapy, have LDL-C levels >140 mg/dl, and in those with rapidly progressive atherosclerotic CVD (defined as recurrent ACS, repeated unplanned coronary revascularizations, or recurrent ischemic stroke, within five years of the index event) and LDL-C levels >100 mg/dl.75
The American College of Cardiology/American Heart Association guideline on the management of blood cholesterol regarding addition of nonstatin therapy to statin therapy specifies that associating ezetimibe or a PCSK9i for secondary prevention should be considered in patients if the target LDL-C reduction of ≥50% has not been achieved (threshold of <70 mg/dl) under maximally tolerated statin therapy. For patients without comorbidities, the first step should be the addition of ezetimibe. However, in patients with a risk-enhancing factor (diabetes, chronic kidney disease, CV event under statin therapy, history of ischemic stroke or MI, age ≥65 years), a PCSK9i should be used instead of ezetimibe if the desired reduction in LDL-C is >25%.76 The more recent American guidelines on the management of hypercholesterolemia support optimizing initial therapy with ezetimibe before considering a PCSK9i, and add a further recommendation to assess the cost-effectiveness of these drugs, analysis of which shows that at 2018 prices in the US, they are “low value”.14 Such considerations, which do not question the results of the clinical trials but instead focus on health gains, will largely depend on the cost of PCSK9i in each country and the incremental cost-effectiveness ratios deemed acceptable within each health system.
Recommendations for PortugalAfter assessing the clinical evidence, cost-effectiveness analyses and the guidelines published by other medical societies, the authors of this document recommend associating a PCSK9i in patients under optimal lipid-lowering therapy who have already suffered an atherothrombotic event and also have:
LDL-C ≥140 mg/dl; or
LDL-C ≥100 mg/dl plus one of the following:
- –
Diabetes with target organ damage such as proteinuria
- –
Polyvascular disease
- –
Significant multivessel coronary disease, with or without revascularization
- –
Recurrent CV event within five years of the index event
- –
FH.
Before considering introducing a PCSK9i, all the following conditions should be met:
- –
Maximally tolerated high-intensity statin therapy (atorvastatin 40-80 mg or rosuvastatin 20-40 mg)
- –
Addition of ezetimibe 10 mg
- –
Adequate control of other risk factors
- –
Implementation of lifestyle changes, including dietary counseling and smoking cessation (Figure 2).
Figure 2.Treatment algorithm for lipid-lowering therapy in patients with a previous atherothrombotic event. CV: cardiovascular; FH: familial hypercholesterolemia; LDL-C: low-density lipoprotein cholesterol; PCSK9i: PCSK9 inhibitor; TOD: target organ damage.
a In patients at very high CV risk (e.g. previous atherothrombotic event plus diabetes, polyvascular disease or recurrent CV event), target LDL-C level should be <55 mg/dl.
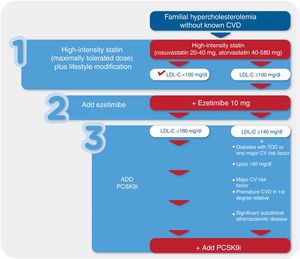
The ESC/EAS guidelines recommend the use of PCSK9i in patients with HeFH without established CVD but at high or very high CV risk if they have LDL-C >180 mg/dl on optimal lipid-lowering therapy.77 They also recommend that in the presence of additional risk factors – diabetes, high Lp(a) levels, premature CVD in a first-degree relative, smoking, and indicators of severity on imaging exams – the LDL-C threshold above which the use of PCSK9i should be considered should be lowered to 140 mg/dl (Figure 3).
PCSK9i are recommended as additional therapy to reduce LDL-C levels in patients with HoFH whether or not they are also undergoing LDL apheresis. However, they are not recommended in patients with two receptor-negative alleles in the LDLR gene, in whom LDLR activity is less than 2%, since the mechanism of action of PCSK9i requires a certain level of LDLR activity.44,54
Recommendations for PortugalIn patients with FH with no previous CV event, associating a PCSK9i should be considered if even after optimal lipid-lowering therapy they have:
LDL-C ≥180 mg/dl; or
LDL-C ≥140 mg/dl plus one of the following:
- –
Diabetes with target organ damage such as proteinuria or one major risk factor
- –
Lp(a) >50 mg/dl
- –
Major risk factors such as uncontrolled stage 2 or 3 hypertension (blood pressure >160/100 mmHg)
- –
Premature CVD in a first-degree relative (men aged <55 years; women aged <60 years)
- –
Significant subclinical atherosclerotic disease.
Again, before considering introducing a PCSK9i, all the following conditions should be met:
- –
Maximally tolerated high-intensity statin therapy (atorvastatin 40-80 mg or rosuvastatin 20-40 mg)
- –
Addition of ezetimibe 10 mg
- –
Adequate control of other risk factors
- –
Implementation of lifestyle changes, including dietary counseling and smoking cessation.
Most cases of statin intolerance are identified by the patient's subjective complaints, while alterations on laboratory tests are a less common reason for discontinuation of statin therapy. Statin intolerance should thus be diagnosed not simply on the basis of the occurrence of symptoms, but only if those symptoms are perceived as intolerable. It is defined as a syndrome that has been verified, confirmed and documented that leads to suboptimal statin dosing, reductions in statin compliance, reduction in patient quality of life, or statin cessation.78,79
There is no universally accepted definition of statin intolerance, but various publications have sought to reach agreement on the diagnostic criteria (Supplementary Table 1). Statin intolerance is commonly held to involve the occurrence of adverse symptoms, almost always musculoskeletal, which the patient perceives as intolerable, and/or the presence of alterations on laboratory testing (such as creatine kinase, alanine aminotransferase/aspartate aminotransferase or bilirubin) suggesting raised risk that are attributable to statins and lead to discontinuation of therapy (Supplementary Table 2). In practice, there is room for improvement in assessing the point at which symptoms become genuinely intolerable, as well as in accurately identifying their cause. Very often, muscle-related and other adverse events are in fact unrelated to statin therapy even though they occur during the course of treatment, and most patients with such symptoms are able to tolerate appropriate dosing levels. Identifying cases of false intolerance is essential to prevent untimely, unnecessary and unwarranted discontinuation of statins by patients who need them.80 Certain characteristics can help the clinician determine the likelihood that statins are in fact causing the symptoms (Supplementary Table 3). The ACC Statin Intolerance app and probability scores81 may also be useful. Factors that increase the risk of statin intolerance or that can trigger symptoms should also be assessed (Supplementary Table 4).
In view of the considerable variability in terms of the number and dosage of statins that patients may be unable to tolerate, in practical terms two categories of intolerance can be defined. Complete statin intolerance is seen when the patient is unable to tolerate at least three different statins, even at their lowest daily dosages (atorvastatin 10 mg, fluvastatin 40 mg, lovastatin 20 mg, pitavastatin 2 mg, pravastatin 40 mg, rosuvastatin 5 mg and simvastatin 20 mg). Partial intolerance, on the other hand, is defined as the inability to tolerate a statin at the dosage needed to attain the desired therapeutic goal. Inability to tolerate certain statins or certain dosages should not lead to an unwarranted diagnosis of statin intolerance that will mean the patient does not receive appropriate treatment.
Although there is agreement that the use of PCSK9i in patients with statin intolerance is logical, there is no formal indication for such use in the current guidelines issued by the European Medicines Agency.
Recommendations for PortugalIn summary, the administration of PCSK9i should be considered for patients who are unable to tolerate appropriate doses of at least three statins, especially if additional risk factors are present such as FH, polyvascular or multivessel disease, or rapidly progressive atherosclerotic CVD.77
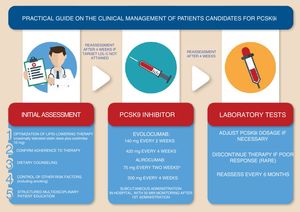
Clinical management of patients under PCSK9 inhibitorsPCSK9i should only be prescribed in a hospital environment under the coordination of specialists in the management of CV risk. Consultations should be based on pre-established diagnostic and therapeutic protocols prepared by a multidisciplinary team.
Eligibility for PCSK9i therapy should be assessed after optimization of pharmacological therapy with maximally tolerated statin doses and ezetimibe, as well as optimization of non-pharmacological therapy, particularly diet guided by a nutritionist and control of other CV risk factors including smoking.6,77 Monitoring of PCSK9i therapy should include verification of adherence to maximally tolerated statins and ezetimibe; the patient's lipid profile should be reassessed after four weeks of full adherence if appropriate. Renal and liver function should be assessed before treatment begins.
Initiation of treatment with a PCSK9i is an opportunity to offer the patient a structured multidisciplinary educational program focusing on atherosclerotic disease and its treatment, using multimedia teaching materials.
The first administration of the drug should take place in hospital and the patient should be monitored for 30 min. Subsequent administrations can be performed at home when the patient or their carers have been duly instructed.
The lipid-lowering effect of the therapy should be assessed around four weeks after beginning treatment. Treatment discontinuation should be considered in poor responders (LDL-C reduction <25%), while dose reduction should be considered if LDL-C levels fall below <25 mg/dl.13
Monitoring of LDL-C levels should be performed at least every six months. Any adverse events should be reported to the National Pharmacovigilance System (Figure 4).
ConclusionPCSK9i are a new therapeutic option that significantly reduces LDL-C levels and CV risk in patients already receiving optimal lipid-lowering therapy.
These guidelines for the use of PCSK9i in clinical practice in Portugal are the result of a multidisciplinary consensus that is centered on prioritizing their use in patients who will derive the greatest benefit: those with the highest risk and the highest LDL-C levels. As with all guidelines, their application in clinical practice cannot replace the clinical assessment of the individual patient.
Conflicts of interestRFC has received consulting or speaker fees from MSD, Bial and Amgen.
PMS has received consulting or speaker fees from Bayer, JABA Recordati, MSD Portugal, Kowa Pharmaceuticals, Novartis, Daiichi Sankyo, Amgen, Sanofi-Regeneron and Tecnimede.
FA reports no conflicts of interest.
CG has received consulting or speaker fees from MSD and Amgen.
JF has received consulting fees from Amgen.
JM has received consulting or speaker fees from Astra Zeneca, Amgen, Bayer, Boehringer Ingelheim, Jaba, Servier, and Novartis.
Please cite this article as: Fontes-Carvalho R, Marques Silva P, Rodrigues E, Araújo F, Gavina C, Ferreira J, et al. Guia prático para a utilização dos inibidores da PCSK9 em Portugal. Rev Port Cardiol. 2019;38:391–405.
Sponsored by the Portuguese Society of Cardiology, the Portuguese Society of Endocrinology, and the Study Group on Vascular Prevention and Risk of the Portuguese Society of Internal Medicine.