We describe the case of a patient with chronic renal failure under hemodialysis for five years who, after renal transplantation, developed acute renal failure and hypertension refractory to medical therapy. Given the clinical and imaging (renal ultrasound and computed tomography) suspicion of renal artery graft thrombosis, invasive angiography was performed, which confirmed the diagnosis. The therapeutic approach consisted of percutaneous thrombus aspiration and subsequent balloon angioplasty of the entire artery, followed by stent implantation in a second procedure. The clinical course was uneventful with improvement of renal function and normalization of blood pressure.
The case highlights the importance of percutaneous intervention in the management of patients with vascular complications after transplantation, with successful application of a procedure normally used in the setting of acute myocardial infarction – percutaneous thrombus aspiration and implantation of a drug-eluting vascular stent.
Descreve-se um caso de um doente com doença renal crónica sob hemodiálise durante cinco anos, que após transplantação renal desenvolveu um quadro de insuficiência renal aguda e hipertensão arterial refractária à terapêutica médica. Pela suspeita clínica e imagiológica (ecografia e tomografia computadorizada renal) de trombose da artéria renal do enxerto, realizou-se angiografia invasiva que confirmou o diagnóstico. A abordagem terapêutica efectuada consistiu em aspiração por via percutânea de material trombótico e subsequente angioplastia por balão em toda a extensão da artéria e em segundo tempo com implantação de um stent. A evolução decorreu sem complicações, com melhoria da função renal e normalização do perfil tensional.
Este caso reforça a importância da intervenção percutânea na abordagem dos doentes com complicações vasculares após transplante, pela aplicação com sucesso de uma técnica habitualmente utilizada no contexto de enfarte agudo do miocárdio – aspiração de trombos por via percutânea e implantação de stent farmacológico vascular.
There have been constant advances in percutaneous endovascular techniques in recent years. Their proven efficacy in various areas, with low rates of complications, has led to a widening of their indications.
Balloon angioplasty, with or without stenting, is frequently used to treat peripheral arterial thrombosis. This approach has an important role in the renal artery bed, particularly in cases of renal artery stenosis after renal transplantation, due to its efficacy and potential clinical impact.
A recently developed technique used in primary angioplasty to treat acute myocardial infarction is thrombectomy by percutaneous manual aspiration of intracoronary thrombi. We describe the case of a patient with renal artery thrombosis after renal transplantation who underwent successful percutaneous thrombus aspiration. Quite apart from its clinical interest, no previous cases have been reported of this technique being performed in the context of renal artery thrombosis after renal transplantation.
Case reportWe present the case of a 64-year-old man with a history of chronic renal disease due to IgA nephropathy, under hemodialysis for five years. He received a right kidney transplant from a cadaver donor in November 2010.
In the immediate post-transplant period, the patient developed oliguria and anuria, necessitating renal function replacement therapy by hemodialysis. Renal ultrasound identified a ureteral obstruction with hydronephrosis. Following implantation of a ureteral stent, diuresis was restored and the pyelocaliceal dilatation was reverted. Renography, which had initially revealed severe hypoperfusion and hypofunction of the transplanted kidney compatible with acute tubular necrosis, showed improved graft perfusion and function after the procedure.
He was discharged a month after transplantation, clinically stable, with serum creatinine 2.9mg/dl.
On emergency re-evaluation a week after discharge, the patient presented signs of heart failure, with severe lower limb edema, orthopnea and persistently high blood pressure (BP) (mean BP>180/90mmHg). Despite diuretic and BP-lowering therapy, his symptoms did not improve, with worsening renal function (peak serum creatinine 8.64mg/dl). Rapid urine testing revealed leukocyturia and microscopic hematuria. Renography showed good perfusion but graft dysfunction. Due to suspicion of transplant rejection, a renal biopsy was performed, which showed incipient acute tubular necrosis. Renal ultrasound revealed allograft renal artery stenosis; computed tomography (CT) enabled better characterization, showing slow uptake of contrast and an endoluminal image interpreted as a thrombus, partially occluding the renal artery.
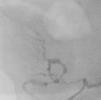
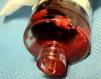
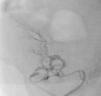
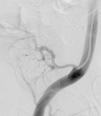
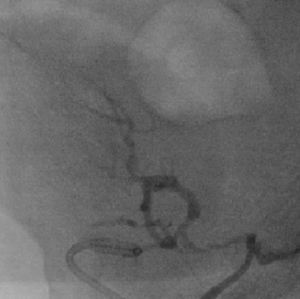
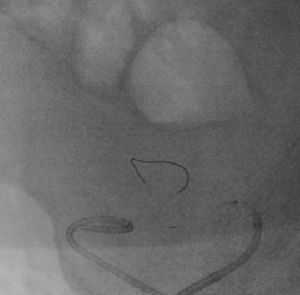
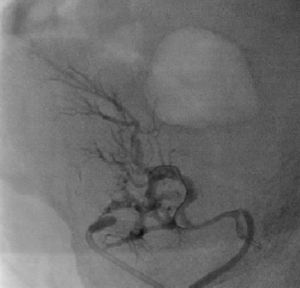
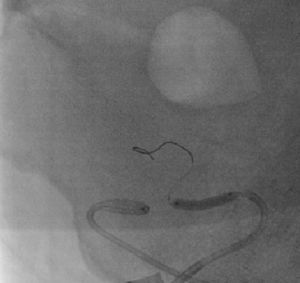
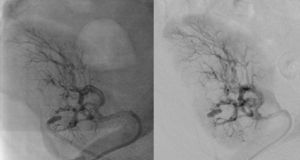
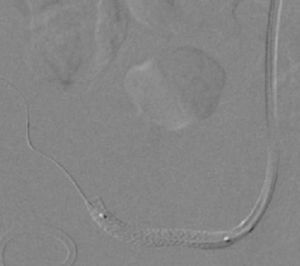
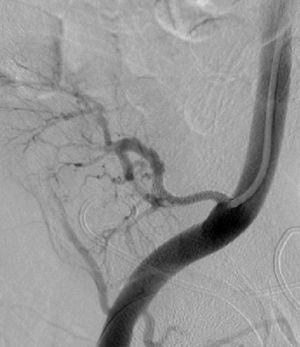
It was therefore decided to perform invasive angiography via right femoral artery access, which showed reduced flow in the renal artery graft and multiple diffuse hyperdense images suggestive of organized thrombi along the entire course of the renal artery of the transplanted kidney (Figure 1). This was followed by selective cannulation of the renal artery using an internal mammary catheter and introduction of a 0.14″ Balance Middleweight Universal II Guide Wire (Abbott Vascular®); the intra-arterial material was aspirated using a Pronto V3 (Vascular Solutions, Inc.®) 6F extraction catheter. Macroscopically the aspirated material was of thrombotic appearance (Figures 2–4). Balloon angioplasty was then performed of the entire renal artery (Figure 5). There was immediate angiographic improvement, and the renal shadow could be visualized, but with slight wall irregularities (Figure 6). Adjuvant pharmacological therapy consisted of aspirin (100mg/day) and unfractionated heparin (5000-U bolus followed by 100U/kg/h) for 72hours, and then oral anticoagulation with warfarin (INR target 2.0–3.0).
The patient rapidly stabilized in clinical and hemodynamic terms, and renal function improved (serum creatinine 2–3mg/dl). Echographic assessment showed the transplanted kidney with regular contours and good corticomedullary differentiation and no dilatation of the pyelocaliceal system.
At 30-day follow-up, worsening of renal function was again observed (serum creatinine 4.09mg/dl); CT angiography was repeated, followed by renal angiography, which showed restenosis. Angioplasty of the allograft renal artery was repeated using the same technique as before, but with implantation of a 3.0mm×28-mm everolimus-eluting Promus Element stent (Boston Scientific®) at 16atm (Figures 7 and 8). The patient's clinical course was favorable after this intervention and there had been no recurrence or other relevant clinical events at three-month follow-up.
DiscussionAllograft renal artery stenosis is a relatively frequent complication after renal transplantation, with an incidence of up to 23%.1 However, there are widely differing definitions of stenosis, due to differences in diagnostic techniques; not all patients undergo renal angiography.2
The main clinical manifestations of renal artery stenosis after renal transplantation are hypertension refractory to medical therapy and graft dysfunction leading to worsening renal function.2–4 Around 1–5% of cases of hypertension and at least 75% of vascular complications after transplantation are attributable to renal artery stenosis.5,6 However, there is a wide clinical spectrum, ranging from asymptomatic disease with hypertension as the only manifestation to acute heart failure.4 Doppler ultrasound screening of renal artery grafts in asymptomatic patients detected renal stenosis in 12.4% of cases.4,7
The presence of an audible murmur in the iliac-femoral region is a common sign but is nonspecific and does not correlate with lesion severity, since there can be critical stenosis with no murmur. The finding may merely reflect physiological turbulence of blood flow close to the anastomosis.1
The main causes of renal artery stenosis after renal transplantation are iatrogenic, mostly related to the surgical technique used at the anastomosis site. Other triggering factors are vessel lesions during preservation or clamping, and torsion or angulation of the artery. Most stenoses are located immediately proximal or distal to the anastomosis, but they can also be diffuse or multiple.1–4 The presence of atherosclerosis in the donor or recipient is an important predisposing factor, mainly associated with late stenosis.8 In the case presented, the angiographic presentation of obliteration of the entire renal artery suggested diffuse atherosclerotic disease of the allograft. Other mechanisms such as immunological causes or local thrombosis due to inadequate flow in the graft are less likely, given the patient's immunosuppressive therapy and good urinary output in the first postoperative month.
Several different imaging techniques have been used in the diagnosis of renal artery stenosis, including Doppler ultrasound, magnetic resonance and CT. Of these, Doppler ultrasound is an invaluable tool due to the wide experience with this technique in post-transplant evaluation, together with good reproducibility and accessibility and the fact that it can be performed at the patient's bedside, essential in intensive care units.2
Nevertheless, invasive arteriography remains the gold standard diagnostic method. On rare occasions it can lead to complications, such as contrast nephropathy with worsening of renal function, distal thromboembolism with irreversible graft loss, arterial dissection and vascular access complications including hematomas, pseudoaneurysms and arteriovenous fístulas.6,9 It is thus not considered a routine screening procedure for transplant renal artery stenosis, but is indicated when there is a high degree of clinical suspicion (refractory hypertension and worsening renal function) on the basis of noninvasive exams.2,4
Although renal artery graft stenosis can be diagnosed early, in most cases the diagnosis is established later, between 3 and 24 months after transplantation.10
The therapeutic approach to transplant renal artery thrombosis can be: (1) conservative, with medical therapy to control BP (short-acting low-dose angiotensin-converting enzyme inhibitors) after excluding decline in renal function or hemodynamic repercussions; (2) percutaneous intervention; or (3) surgical.9,11 A few cases of spontaneous regression of arterial stenosis have been reported.
Percutaneous intervention with balloon angioplasty is the first-line treatment in most patients. The technique has been thoroughly validated for treating coronary stenosis, and its application in other contexts including transplant renal artery stenosis is associated with high success rates; immediate success is over 90%,12–14 and arterial patency after five years is over 85%.15 It has greater efficacy in short linear lesions distal to the anastomosis.9 However, the rate of recurrence can reach 30% at 6–8 months if performed without stent implantation, as opposed to less than 10% in combined balloon and stent angioplasty.12,16
The use of drug-eluting stents in this context has not been described. In the case reported, the initial approach deliberately did not include implantation of a stent due to the increased amount of contrast this would require in a case of obstructive renal failure and the fact that it could result in reduced flow. This innovative solution was subsequently adopted as it was considered the most appropriate given the favorable clinical course following the initial procedure and restenosis after balloon angioplasty despite immunosuppression with oral sirolimus. The choice of stent diameter was guided angiographically since there was insufficient guidewire length to perform intravascular ultrasound.
Surgery is an option in cases of unsuccessful angioplasty or of severe lesions that cannot be accessed percutaneously.3 Success rates range between 63% and 92%, with a recurrence rate of 12%, but complications are more frequent and severe than for percutaneous intervention.10,17
There are no defined clinical parameters to monitor renal perfusion, including for BP and serum creatinine levels. Nevertheless, there is early improvement in these parameters after percutaneous treatment of renal stenoses.14 Interventions are assessed and monitored by Doppler ultrasound.
In the case presented, a setting suggestive of renal artery graft stenosis began around 40 days after renal transplantation, with a typical presentation of hypertension refractory to medical therapy and acute renal failure. Noninvasive imaging techniques supported this diagnosis, confirmed by invasive angiography, which enabled characterization of the extent and severity of the lesions (multiple and diffuse stenoses along the entire course of the transplant renal artery) as well as the type of lesion (digital subtraction images suggestive of intra-arterial thrombotic material). The initial treatment option was mechanical thrombectomy, as used in acute myocardial infarction, which was uneventful and confirmed the presence of thrombi, followed by balloon angioplasty of the entire renal artery.
Intra-arterial thrombi can result in distal embolization and the no-reflow phenomenon, which is responsible for microvascular obstruction and reduced myocardial perfusion in acute myocardial infarction. Various techniques have been developed to prevent such embolization and protect the microcirculation in this context, including percutaneous thrombus aspiration.18 This technique has been prospectively assessed in ST-segment elevation myocardial infarction, and is associated with reduction in clinical and angiographic events.19 Given the pathophysiological mechanisms underlying intra-arterial thrombi, it can be assumed that its impact will be similar in other arterial beds, and it is thus a promising technique in these cases.
Despite recent advances in percutaneous intervention for renal artery stenosis in transplant recipients, some questions remain: how to determine whether a stenosis is significant; what is the long-term outcome after revascularization; and how and when to monitor interventions during follow-up.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Dores, H. Aspiração de trombos por via percutânea em estenose da artéria renal após transplante renal. Rev Port Cardiol. 2012. http://dx.doi.org/10.1016/j.repc.2012.04.017.