Reentrant circuits of ventricular tachycardia may involve not only the endocardium but also the epicardium. Epicardial ablation can be useful in these situations.
ObjectiveThe aim of this study was to assess efficacy, safety and complications in a series of consecutive patients who underwent ablation of ventricular tachycardia with epicardial mapping.
MethodsThe study included all patients undergoing ventricular tachycardia ablation with epicardial mapping from 2004 to 2012. Of a total of 95 ablations, an epicardial approach was attempted in nine patients, eight male, mean age 58±12 years. Endocardial mapping was performed in all patients previously or simultaneously. The etiology of the arrhythmia was non-ischemic in eight patients and ischemic in one. We compared the number of events in the six months prior to the epicardial procedure and six months after.
ResultsPercutaneous epicardial access was achieved in eight patients. In one case it was not possible due to the presence of adhesions. In none of the patients was the procedure repeated and there were no major complications during hospitalization. In a mean follow-up of 3.5±1.2 years, one patient suffered stroke; there were no other medium-to-long-term complications and the number of ventricular tachycardia episodes was reduced in all patients after ablation.
ConclusionsEpicardial radiofrequency ablation of ventricular tachycardia was effective in reducing morbidity in eight patients, with a low risk of complications in the short and medium-to-long term.
Os circuitos de reentrada da taquicardia ventricular envolvem, por vezes, não só o endocárdio, mas também o epicárdio. A ablação de taquicardia ventricular por via epicárdica pode ser útil nessas situações.
ObjetivoO objetivo do estudo consistiu em avaliar a eficácia, segurança e complicações de uma série doentes consecutivos submetidos a ablação de arritmias ventriculares com mapeamento por via epicárdica.
População e métodosForam incluídos no estudo todos os doentes submetidos a ablação de taquicardia ventricular com mapeamento por via epicárdica desde 2004 até 2012. De um total de 95 ablações, em nove doentes foi tentada a via epicárdica, oito do sexo masculino com 58 ± 12 anos. Todos os doentes tinham sido previamente submetidos ou realizaram concomitantemente mapeamento endocárdico. A etiologia da arritmia era não isquémica em oito doentes e isquémica num. Comparou-se o número de eventos nos seis meses anteriores ao procedimento epicárdico com os seis meses seguintes.
ResultadosO acesso epicárdico foi conseguido em oito doentes por via percutânea subxifoideia. Num caso não foi possível abordagem epicárdica por presença de aderências. Em nenhum dos doentes o procedimento foi repetido e não se verificaram complicações major no período intra-hospitalar. Num seguimento médio de 3,5 ± 1,2 anos registou-se um acidente vascular cerebral num doente, não se registando outras complicações a médio/longo prazo, tendo a maioria dos doentes diminuído o número de episódios de taquicardia ventricular após ablação.
ConclusõesA ablação de taquicardia ventricular por via epicárdica foi utilizada eficazmente na diminuição da morbilidade em oito doentes com baixo risco de complicações a médio/longo prazo.
Ventricular arrhythmias are a leading cause of morbidity and sudden death. Treatment can include medical therapy, implantable cardioverter-defibrillators (ICDs) and ablation. ICDs are effective in interrupting sustained ventricular tachycardia (VT) or ventricular fibrillation (VF), and thus in preventing sudden death. Nevertheless, they do not alter the underlying arrhythmogenic substrate.1 Ablation by the application of energy is the only option to eliminate or modify VT circuits.
Percutaneous ablation is a useful technique for the treatment of VT in patients with structural heart disease or in those with ICDs receiving multiple shocks. It was first attempted in 1983,2 since when significant advances have been made. Success rates in structural heart disease range between 50% and 70% in different series,3,4 and depend on various factors including the center's experience, the ability to identify the circuit responsible and the etiology of the underlying disease.
Ablation usually consists of the application of radio-frequency energy in the area of the endocardium shown by mapping to be responsible for sustaining the arrhythmia. The rate of VT recurrence after endocardial ablation in patients with ischemic heart disease (IHD) is approximately 50%.5,6 One limitation of this approach is difficulty in locating the reentrant circuit; at times, it is impossible to treat the critical zone of the circuit if this is located in the subendocardium or epicardium. Epicardial circuits are identified in 10–30% of patients with IHD and in over 30% of those with non-ischemic VT.7,8
Epicardial mapping and ablation were initially only possible by cardiac surgery, but in 1996, Sosa et al. described a percutaneous technique.9
ObjectiveThe aim of this study is to describe the experience of the Arrhythmia Unit of the Cardiology Department of Hospital de Santa Cruz in the epicardial mapping and ablation of ventricular arrhythmias, in what we believe is the first series reported in Portugal.
MethodsOf a total of 95 VT ablations in our institution from 2004 to 2012, percutaneous epicardial access was attempted in nine patients, eight male, mean age 58±12 years. Only two patients presented cardiovascular risk factors (hypertension and dyslipidemia) and none had serious comorbidities such as chronic renal failure, pulmonary disease or peripheral arterial disease. Most had been diagnosed with dilated cardiomyopathy (DCM) and had previously undergone endocardial ablation. Five patients had ICDs, three for primary and two for secondary prevention.
ProcedureProcedures were performed under conscious sedation with a perfusion of propofol 2 mg/kg/h and remifentanil <0.2 μg/kg/min in all cases, adjusted to the specific needs of each patient. Patients’ O2 Sat and expired CO2 levels were monitored by capnography. In all cases, catheters were placed in the coronary sinus and the right ventricle, and withdrawn to the most proximal area when a His bundle electrogram was required. Access to the epicardial space was not possible in one patient due to the presence of adhesions; in six patients specifically referred for an epicardial procedure following recurrent VT or a failed first procedure it was achieved at the first attempt, while in two it was performed after endocardial mapping during the procedure suggested an epicardial origin.
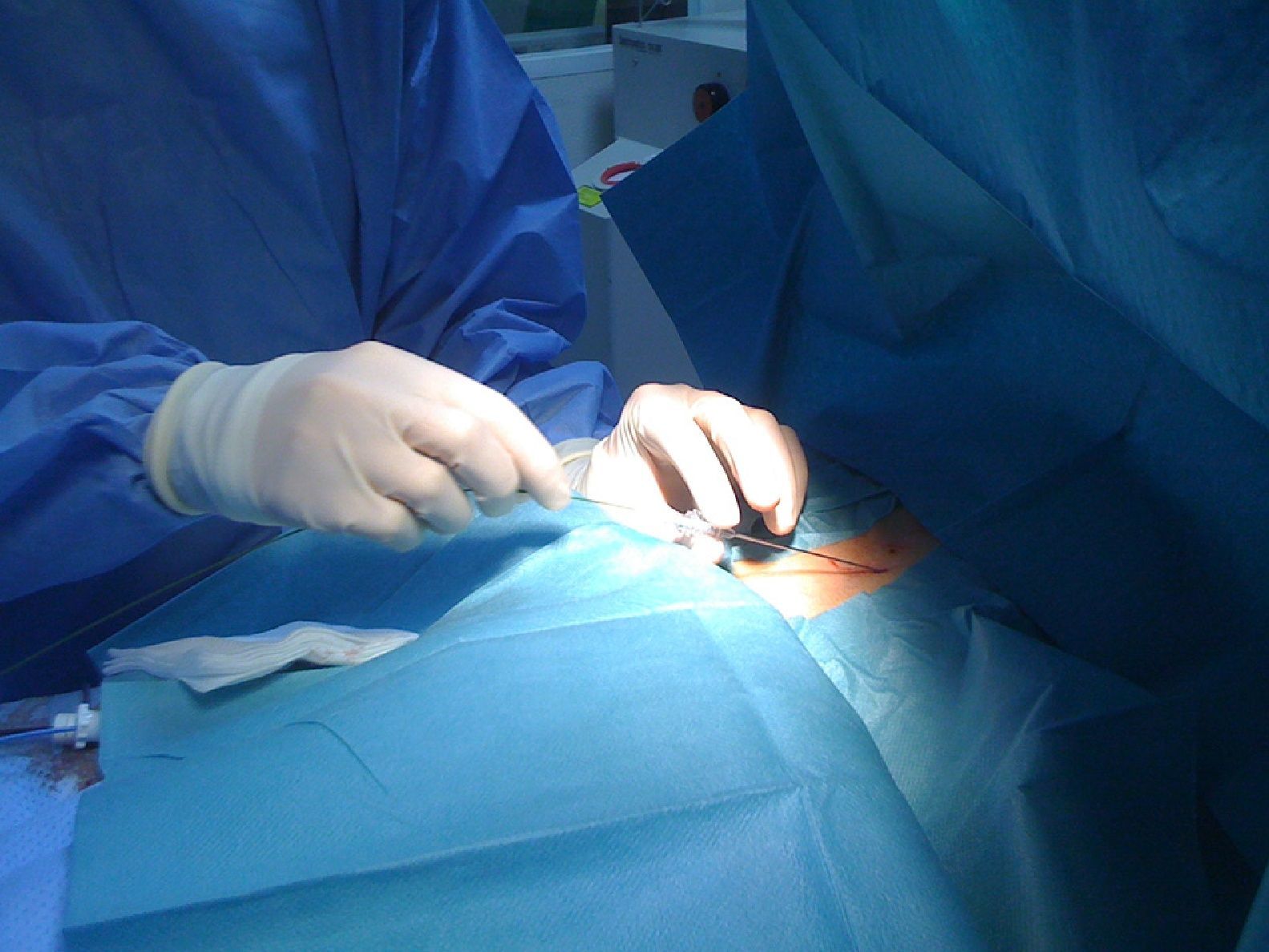
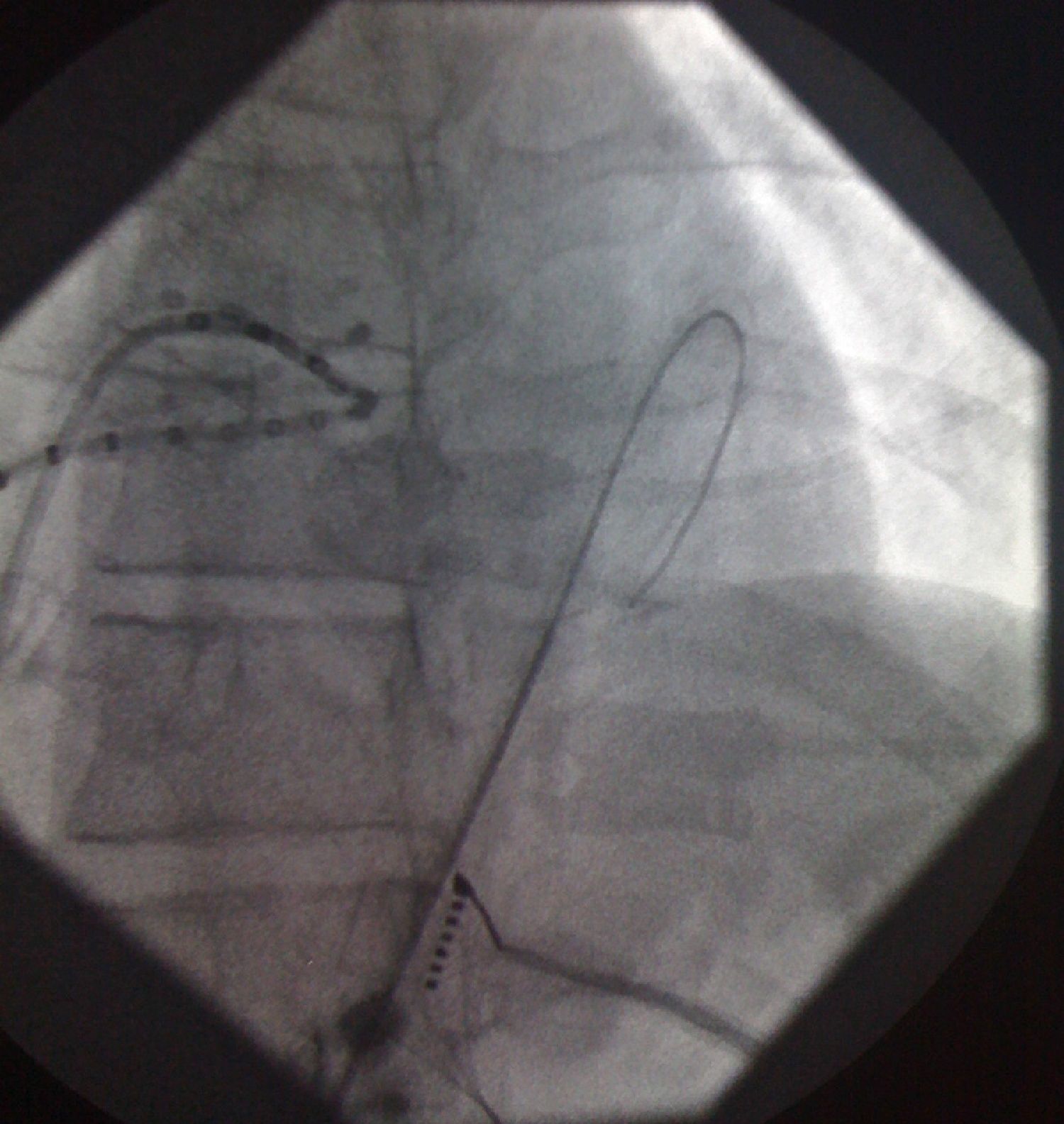
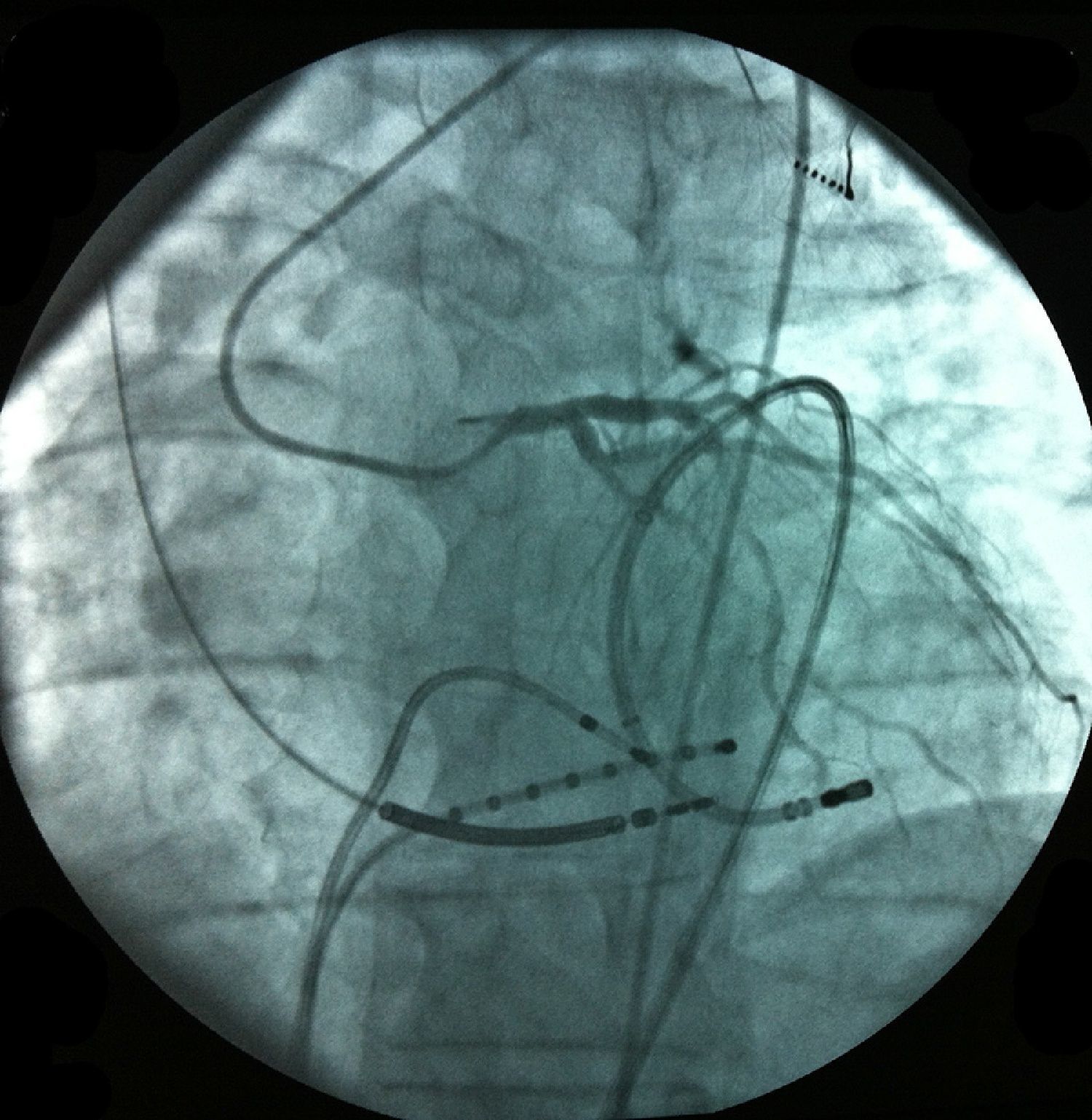
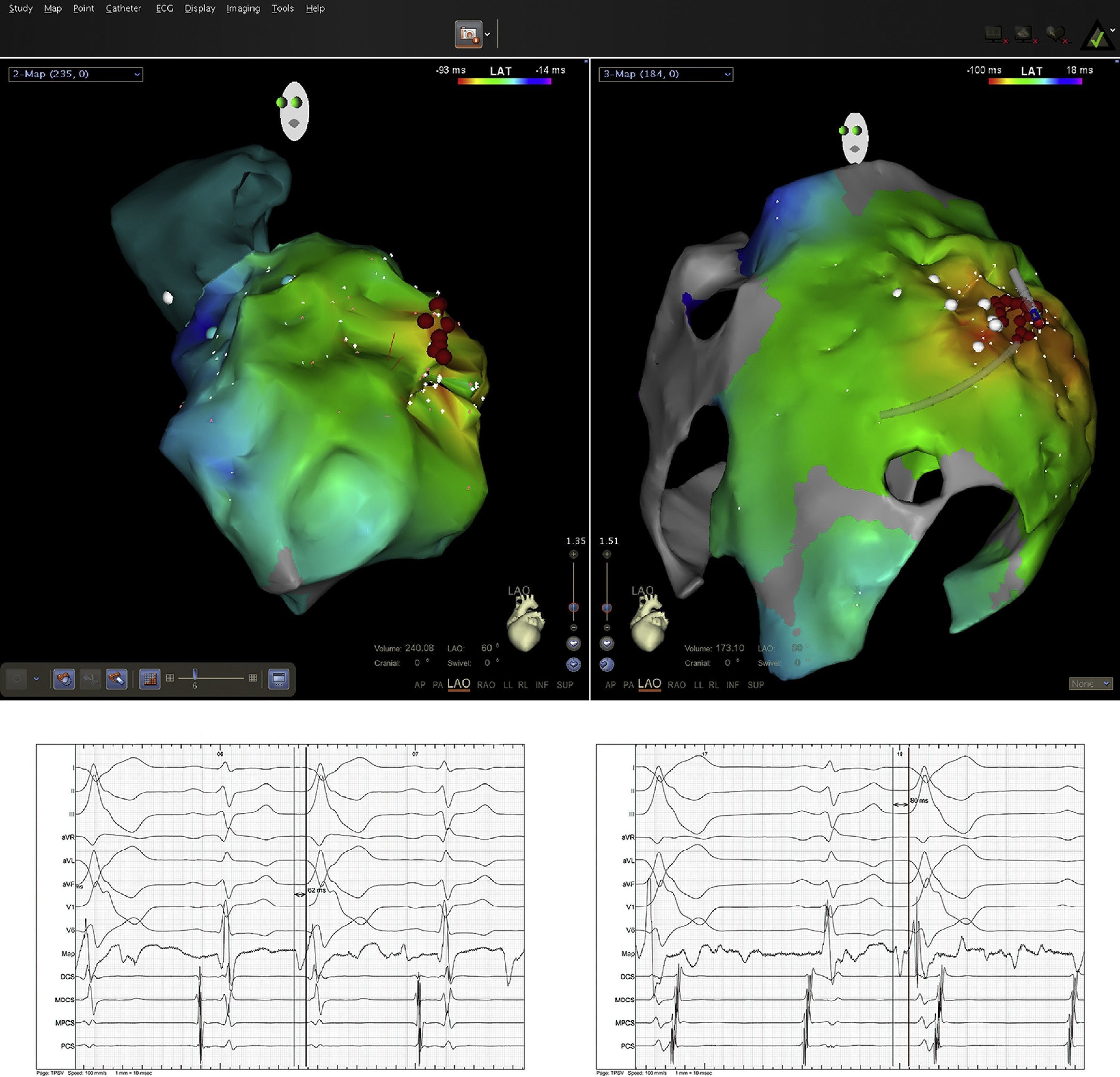
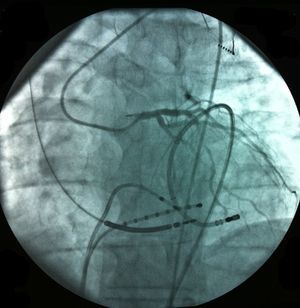
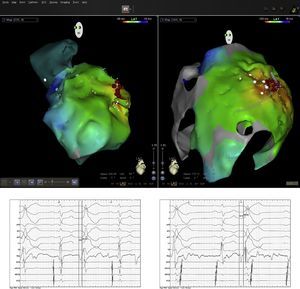
Pericardial access was obtained through percutaneous subxiphoid puncture with a Tuohy needle (or pericardiocentesis needle) as described by Sosa et al.9,10 The puncture was made between the left margin of the xiphoid appendix and the lower margin of the adjacent rib with the needle directed towards the left shoulder (Figure 1). Correct positioning was confirmed by visualization of a small quantity of contrast (∼1 ml) in the epicardial space, after which a guidewire was inserted (Figure 2). An introducer was passed over the guidewire, followed by the ablation catheter.10 After access to the epicardial space was obtained, intravenous heparin (100 U/kg) was administered to enable coronary angiography and endocardial ablation to be performed safely. Once within the epicardial space, the catheter can be manipulated to map the entire epicardial surface11 (Figures 3 and 4). A 4-mm catheter was used in the first two cases, while an irrigated catheter was used in the other six. In all cases the ablation strategy was to identify the critical isthmus involved in sustaining the tachycardia with the aid of electroanatomical mapping (CARTO system, Biosense Webster). The radiofrequency parameters employed depended on the type of catheter – 20–50 W for the 4-mm catheter and 15–30 W for the irrigated catheter (8–15 cc/min). During the procedure, the epicardial space was drained using a pigtail catheter for set periods of approximately 15 min, adjusted to the needs of the patient, and after the procedure, by means of an introducer for occasional aspiration over a maximum period of three hours. No patient required corticosteroids or anti-inflammatory drugs.
The mapping and ablation techniques were similar to those used in the endocardial space, although entrainment maneuvers are more difficult using bipolar pacing due to the high epicardial stimulation threshold. On electroanatomical mapping, areas with bipolar voltage of less than 0.1 mV were considered to be scar tissue. All patients with monomorphic VT with different morphologies had one clinically dominant morphology, which was selected for ablation.
Patients were hospitalized for 24 hours. Post-procedure echocardiography was performed only in patients with suggestive symptoms; none presented pericardial effusion. All patients underwent coronary angiography prior to radio-frequency ablation to assess proximity to the coronary arteries (energy was applied only >5 mm from a coronary artery). During the procedures, pacing (28 mA at 2 ms) was performed along the left ventricular lateral wall to capture the left phrenic nerve on three-dimensional mapping in order to avoid injury during energy application.
We compared the number of events in the six months prior to the epicardial procedure and six months after. Arrhythmic events and ICD shocks were recorded, mainly through device interrogation but also through ECG and 24-hour Holter monitoring.
ResultsPercutaneous subxiphoid access was achieved in eight patients. Epicardial ablation was performed in six as second-line therapy after previous endocardial procedures failed and as a combined procedure in two. The etiology of the arrhythmia was non-ischemic in seven and ischemic in one. In the four patients with idiopathic DCM, posteroseptal, posterolateral or anterolateral scars were identified by voltage criteria in all cases; one Brazilian immigrant living in Portugal for five years had Chagas disease, one patient had IHD in the dilated phase with significant left ventricular dysfunction, one had tachycardiomyopathy, and one had symptomatic frequent ventricular extrasystoles (24730 on 24-hour Holter ECG) of the right ventricular outflow tract. All patients were under antiarrhythmic therapy and in NYHA class II or III, and five had an ICD (Table 1). No patient had undergone cardiac surgery.
Characteristics of population undergoing epicardial ablation.
| Gender | Underlying disease | ICD at time of ablation | Previous endocardial ablation | Type of arrhythmia | Cardiovascular risk factors | Ejection fraction | NYHA at time of ablation | |
| 1 | M | Idiopathic DCM | Yes | 3 | Monomorphic VT (>1 morphology) | Yes | <35% | III |
| 2 | M | Idiopathic DCM | Yes | 1 | Monomorphic VT (>1 morphology) | No | 40% | II |
| 3 | M | Ischemiccardiomyopathy | Yes | No | Monomorphic VT | Yes | <35% | II |
| 4 | M | Idiopathic DCM | Yes | 1 | Monomorphic VT | No | <35% | II |
| 5 | M | Idiopathic DCM | No | 1 | Monomorphic VT (>1 morphology) | No | 42% | II |
| 6 | M | RVOT VES | No | No | VES | No | >55% | – |
| 7 | F | Chagas disease | Yes | 1 | Monomorphic VT | No | 45% | – |
| 8 | M | Tachycardiomyopathy | No | 2 | Monomorphic VT | No | 42% | III |
DCM: dilated cardiomyopathy; F: female; ICD: implantable cardioverter-defibrillator; M: male; NYHA: New York Heart Association class; RVOT: right ventricular outflow tract; VES: ventricular extrasystoles; VT: ventricular tachycardia.
In one case, the presence of adhesions prevented epicardial access and so the procedure was halted. In the patient with frequent ventricular extrasystoles, the application of radiofrequency energy was ineffective, probably due to the presence of epicardial fat. Of the eight patients in whom epicardial access was achieved, six underwent epicardial ablation. On average, two VTs were induced per patient, epicardial circuits being identified in 75%. In seven cases (six during application in the pericardial space), the VT was interrupted during radiofrequency application and could not be re-induced, an immediate success rate of 87.5%. The patient with Chagas disease was diagnosed with stroke following an episode of left hemiparesis one week after a combined ablation procedure, with no sequelae. There were no other procedure-related complications.
Follow-upAll patients were alive after a mean follow-up of 3.5±1.2 years (six months to six years). Comparison of the number of events in the six months prior to the procedure and six months after showed a decrease in the number of VT episodes and ICD shocks. In the four patients with DCM, two were free of sustained VT episodes during follow-up, and in the other two there was a significant reduction not only in arrhythmic events but also in ICD shocks. The patient with Chagas disease had no new episodes of sustained VT following epicardial ablation. No significant symptomatic relief was achieved in the patient with frequent ventricular extrasystoles, probably due to the presence of epicardial fat. The patient with tachycardiomyopathy is event-free, with improved ejection fraction (>55%), and the patient with IHD has had a reduced number of events. In those with longer follow-up, the trend for fewer events has been maintained (Table 2).
Results before and after epicardial ablation.
| Disease | Gender | RF application | VT before (6 months before) | VT after (6 months after) | Shocks before (6 months before) | Shocks after (6 months after) |
| DCM | M | Epi/Endo | >10 (>10) | NSVT | 5 (5) | 0 |
| DCM | M | Epi/Endo | >10 (8) | 2 (2) | 4 (2) | 0 |
| DCM | M | Epi/Endo | >10 (>10) | 3 (2) | 4 (3) | 1 (1) |
| DCM | M | Endo | 2 (2) | 0 | – | – |
| TCM | M | Epi/Endo | 3 (3) | 0 | – | – |
| Ischemic DCM | M | Endo | 5 (3) | 1 | 3 (3) | 1 (1) |
| RVOT VES | M | Epi/Endo | – | – | – | – |
| Chagas | F | Epi/Endo | 3 (2) | NSVT | 2 (1) | 0 |
DCM: dilated cardiomyopathy; Endo: endocardial; Epi: epicardial; F: female; M: male; NSVT: non-sustained ventricular tachycardia; RF: radiofrequency; RVOT VES: right ventricular outflow tract ventricular extrasystoles; TCM: tachycardiomyopathy; VT: ventricular tachycardia.
The two patients with the longest follow-up (six years) presented no long-term complications secondary to the procedure.
DiscussionThere was a high success rate of symptom relief in our study population, with a low rate of complications. Despite the complications reported in other studies and multicenter analyses (5% major complications in the acute phase and 2% in the long term),14 the risk appears justified in selected cases since there is no alternative treatment for epicardial reentrant circuits. The main underlying diseases associated with this type of circuit are non-ischemic DCM, followed by right ventricular arrhythmogenic dysplasia, and ischemic cardiomyopathy.7 Careful patient selection is essential to avoid complications, since patients who have undergone previous cardiac surgery (a relative contraindication), valve surgery or coronary revascularization present a greater risk of adhesions and difficulty in achieving access to the pericardial space, and therefore a higher risk of right ventricular puncture or other complications. Use of a Tuohy needle, contrast injection and long guidewires that ensure that no cardiac chamber is perforated increase the efficacy of the procedure and, more importantly, significantly reduce the risk of complications.5 Since percutaneous epicardial VT ablation is a relatively recent technique, there have been no studies as yet on possible adverse coronary effects in the long term. Nevertheless, there is agreement that a good compromise between risk and benefit is achieved when the radiofrequency energy is applied at a distance of least 5 mm from the coronary arteries.5 Due to the risk of intimal hyperplasia and thrombosis, application in the vicinity of the coronary arteries should be performed with caution.12,13 There was no case of myocardial infarction in our study population.
The greatest risk when undertaking subxiphoid puncture to access the epicardial space is of perforating intraperitoneal structures such as the liver, stomach or large intestine, especially in patients with DCM or hepatomegaly, but none of these complications occurred in our series.
Other possible complications, with rates of less than 1%,14 include bleeding, pericardial effusion or tamponade and ventricular wall rupture, which were also not seen in our population.
The substrate for the development of sustained monomorphic VT is areas of ventricular scarring that promote reentrant circuits. In IHD, these areas of fibrosis are generally found in the endocardium, which facilitates ablation, explaining the greater success achieved in such cases. In non-ischemic DCM, low-voltage areas are more difficult to identify on electroanatomical mapping, as at times they are not in the endocardium, which complicates the procedure and often requires a different approach.8 In the cases of idiopathic DCM in our series, voltage mapping was able to identify scarring in the posteroseptal, posterolateral and anteroseptal regions, the latter in one case only. Unlike in IHD, in which most infarct scars are clearly located in the endocardial wall,15 DCM most often presents with subendocardial or epicardial scarring. In these cases, identifying the scar responsible for the tachycardia for the purposes of ablation may only be possible via an epicardial approach.16 In Chagas disease a hybrid epicardial and endocardial approach should be adopted from the outset, given that there is a 70% probability of an epicardial circuit.17 However, given the small number of patients with Chagas disease, such cases are not high on the list of interventions. Despite advances in epicardial voltage mapping techniques, percutaneous ablation of tachycardia of ischemic etiology with a single endocardial substrate still has higher success rates.16,18
The presence of epicardial fat affected the efficacy of radiofrequency ablation in one of our patients. This is a common problem, more frequently seen in patients with coronary disease, that reduces the procedure's efficacy, even with irrigated catheters.19,20
The question remains as to which patients will benefit most from the technique. Various criteria have been proposed to raise the level of suspicion of an epicardial origin of an arrhythmia, all of which are based on the principle that conduction velocities are slower when the circuit is in the epicardial region (giving rise to initial slurring of the QRS) than when the endocardial surface is involved. These criteria (for example a pseudo delta wave during the VT) do not have high sensitivity or specificity,21 and it is debatable whether they are of value.22,23 In our series, we selected patients in whom endocardial ablation had already been attempted, irrespective of QRS morphology during tachycardia. Given the high prevalence of epicardial scarring in Chagas disease, an epicardial approach was chosen from the outset in this patient.
ConclusionsThere are clear advantages of an epicardial approach to VT ablation in selected patients, with a low risk of complications.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Galvão Santos P, Cavaco D, Adragão P, et al. Ablação epicárdica percutânea em arritmias ventriculares. Rev Port Cardiol. 2014;33:273–279.