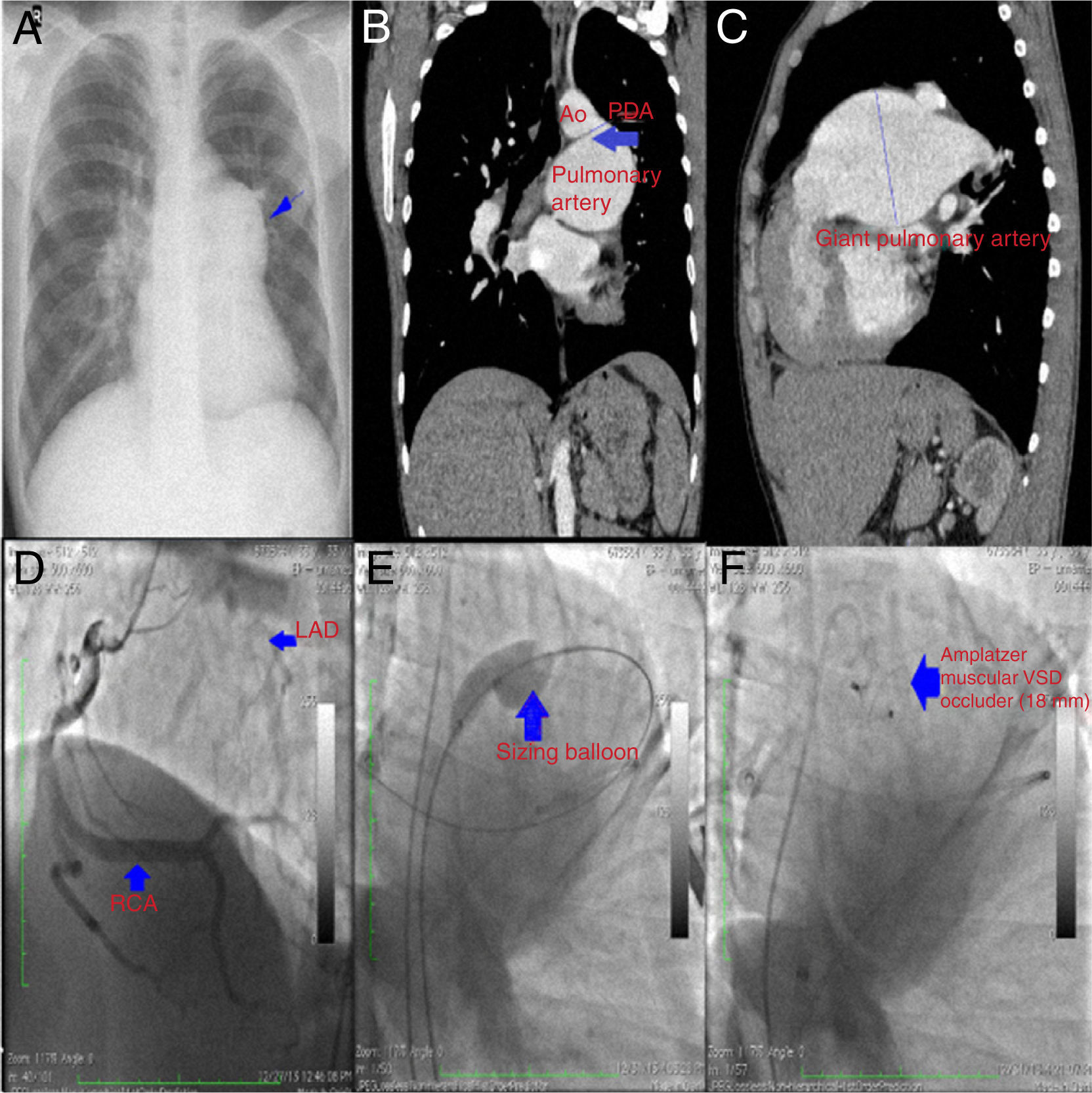
A large patent ductus arteriosus in a patient with a giant pulmonary artery and congenital single coronary artery is a rare congenital cardiovascular malformation. In this report, we present images and videos of the percutaneous closure of an unusually large patent ductus arteriosus in a 33-year-old man with high pulmonary artery pressure.
A 33-year-old man was diagnosed with a patent ductus arteriosus (PDA) shortly after birth, but was then lost to follow-up. He had no symptoms and received no medical treatment until he presented with a one-month history of progressive dyspnea and palpitations. On physical examination he had a prominent left ventricular impulse with a loud continuous murmur. There was no evidence of cyanosis, clubbing, or peripheral edema. His chest X-ray showed cardiomegaly and enlargement of the left pulmonary hilum, and an electrocardiogram revealed sinus tachycardia with incomplete right bundle branch block (Figure 1A). A transthoracic echocardiogram revealed a PDA with left-to-right shunt (pulmonary/systemic flow [Qp/Qs] ratio of 1.6), left ventricular ejection fraction of 60% and enlargement of the right heart chambers and the left pulmonary artery, in addition to severe pulmonary hypertension. Contrast computed tomography revealed a PDA (approximately 16 mm in diameter at its narrowest portion) connecting the aortic isthmus (immediately distal and inferior to the left subclavian takeoff) to the main pulmonary artery and coexistent with a giant left pulmonary artery (80.32 mm) (Figure 1B and C). Coronary angiography showed a single coronary artery that originated from the right coronary artery (Figure 1D and Videos 1 and 2). During cardiac catheterization, peak systolic pulmonary artery pressure was measured at 110 mmHg and pulmonary vascular resistance was 4.8 Wood units. Vasoreactivity testing with adenosine was negative. A sizing balloon (Amplatzer Sizing Balloon II, St. Jude Medical, USA), diameter 20 mm, was used and the duct was measured at 17 mm in length by 6 mm in width at its narrowest diameter (Figure 1E). A fall in systolic pulmonary artery pressure of more than 30% during balloon occlusion was our criterion for proceeding to transcatheter closure (Video 3). The PDA was subsequently successfully repaired using an 18 mm Amplatzer muscular ventricular septal defect occluder (AMVSD) (St. Jude Medical, St. Paul, MN, USA) (Figure 1F and Video 4). A repeat echocardiogram after the procedure at one-year follow-up showed no evidence of residual shunting and progressive decreases in pulmonary artery diameter (71 mm) and right ventricular chamber diameters and pressures.
(A) Chest X-ray showing cardiomegaly and enlargement of the left pulmonary hilum (blue arrow); (B) computed tomography (CT) image revealing a patent ductus arteriosus (PDA) in the frontal plane (blue arrow); (C) CT image showing a giant left pulmonary artery (8.32 cm); (D) coronary angiography showing a single coronary artery originating from the right coronary artery; (E) sizing balloon measuring the diameter of the PDA; (F) the duct is occluded by an Amplatzer muscular ventricular septal defect occluder.
In conclusion, off-label use of the AMVSD may be considered for the occlusion of large PDAs in adult patients with high pulmonary artery pressure.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial supportThis research received no specific grant from any funding agency, either commercial or not-for-profit.
Conflicts of interestThe authors have no conflicts of interest to declare.