Aortic stenosis (AS) is a complex systemic valvular and vascular disease with a high prevalence in developed countries. The new entity “paradoxical low-flow, low-gradient aortic stenosis” refers to cases in which patients have severe AS based on assessment of aortic valve area (AVA) (≤1 cm2) or indexed AVA (≤0.6 cm2/m2), but paradoxically have a low mean transvalvular gradient (<40 mmHg) and a low stroke volume index (≤35 ml/m2), despite preserved left ventricular ejection fraction (>50%).
A search was carried out in the PubMed database on paradoxical AS for the period 2007-2014. A total of 57 articles were included for this review. The prevalence of paradoxical AS ranged from 3% to 35% of the population with severe degenerative AS. It was more frequent in females and in older patients. Paradoxical AS was associated with characteristic left ventricular remodeling as well as an increase in systemic arterial stiffness. It was noted that there may be errors and inaccuracies in the calculation of AVA by the continuity equation, which could erroneously suggest the paradoxical phenotype. There are new diagnostic methods to facilitate the study of AS, such as aortic valve calcium score, valvuloarterial impedance and the longitudinal mechanics of the left ventricle. With regard to its natural history, it is not clear whether paradoxical AS corresponds to an advance stage of the disease or if paradoxical AS patients have a distinct phenotype with specific characteristics. Valve replacement, either surgical or percutaneous, may be indicated in patients with severe and symptomatic paradoxical AS.
A estenose aórtica (EA) é uma doença valvular e vascular sistémica, com elevada prevalência nos países desenvolvidos. A nova entidade «EA grave paradoxal, baixo fluxo/baixo gradiente» refere-se aos casos em que os doentes apresentam EA grave com base na avaliação da área valvular aórtica (AVA) (≤1cm2) ou AVA indexada (≤0,6cm2/m2), mas que paradoxalmente tenham um gradiente médio transvalvular baixo (<40mmHg), com baixo volume de ejeção sistólico indexado (≤35ml/m2), apesar de uma fração de ejeção do ventrículo esquerdo preservada (>50%).
Foi realizada uma pesquisa através da base de dados da PubMed sobre a EA paradoxal no período de 2007-2014. Para a presente revisão foram incluídos um total de 57 artigos.
A prevalência da EA paradoxal variou entre 3-35% da população com EA degenerativa grave. Foi mais frequente no género feminino e nos doentes com idade mais avançada, e esteve associada a uma remodelagem característica do ventrículo esquerdo, bem como a um aumento da rigidez vascular arterial sistémica. Assinala-se a possibilidade de erros e imprecisões no cálculo da AVA pela equação da continuidade, que podem sugerir o fenótipo paradoxal. Existem outros métodos de diagnóstico que podem auxiliar no estudo da EA, como o score de cálcio, a avaliação da impedância valvuloarterial e o estudo da mecânica longitudinal do ventrículo esquerdo. Relativamente à história natural, não é claro que a EA paradoxal corresponda a uma fase avançada da doença valvular aórtica, ou se representa um fenótipo distinto com especificidades próprias. A terapêutica de substituição valvular, cirúrgica ou percutânea, pode estar indicada no doente com EA paradoxal grave e sintomática.
atrial fibrillation
aortic stenosis
aortic valve area
indexed aortic valve area
projected aortic valve area
brain natriuretic peptide
blood pressure
body surface area
coronary artery disease
computed tomography
energy loss coefficient
left ventricular global longitudinal strain
heart rate
low-flow, low-gradient aortic stenosis
left ventricular
left ventricular ejection fraction
left ventricular outflow tract
mean pressure gradient between aorta and left ventricle
peak flow velocity
peripheral vascular resistance
systemic arterial compliance
surgical aortic valve replacement
stroke volume index
transcatheter aortic valve replacement
valvuloarterial impedance
Aortic stenosis (AS) is the most common valve disease in Europe, and is most often due to degenerative etiology.1 It is estimated to affect 2-7% of the population aged over 65 years.2 Degenerative AS progresses slowly and is associated with various clinical manifestations including angina, syncope, cardiac rhythm disturbances, heart failure and sudden death.1
According to the European and American guidelines on valvular disease, severe AS is defined as aortic valve area (AVA) of ≤1 cm2, mean pressure gradient (MPG) between aorta and left ventricle of ≥40 mmHg, or peak flow velocity (PFV) of ≥4 m/s, with normal cardiac output.1,3
In the absence of valve replacement, AS evolves to a form of heart failure with left ventricular (LV) dilatation and dysfunction. In this stage there is a reduction in transvalvular gradients, caused by diminished systolic flow through the aortic valve due to impaired LV systolic function. This entity, first described by Carabello et al. in 1980,4 is termed low-flow, low-gradient aortic stenosis (LF-LG-AS). It is characterized by an AVA of ≤1 cm2 or indexed AVA (AVAi) of ≤0.6 cm2/m2, MPG of <40 mmHg and left ventricular ejection fraction (LVEF) of ≤40%. Patients with LF-LG-AS account for only 5-10% of those with severe AS.5–7
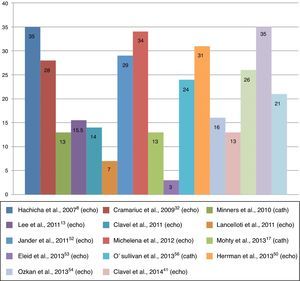
In 2007, Hachicha et al.8 described for the first time a form of AS with reduced systolic flow but, paradoxically, preserved LVEF. This new entity, which they termed paradoxical low-flow, low-gradient aortic stenosis, was defined as AVA of ≤1 cm2, MPG of <40 mmHg, and stroke volume index (SVI) of ≤35 ml/m2, despite LVEF of ≤50%.3,5,9 This entity has been shown to have a significant prevalence and it is thought that it may represent an advanced form of AS.8,10
The aim of the present article was to perform a systematic review of the literature on paradoxical AS, discussing aspects of definition, clinical characteristics, diagnosis, prognosis, natural history, and treatment.
MethodsA search was carried out in the PubMed database up to December 29, 2014, using the following search terms: “Paradoxical aortic valve stenosis” and one of: “low flow”, “low gradient”, “assessment”, “left ventricular ejection fraction”, “echocardiography”, “treatment”, or “prognosis”. The search covered articles in English published since 2007 and included original articles, clinical trials, reviews and animal trials.
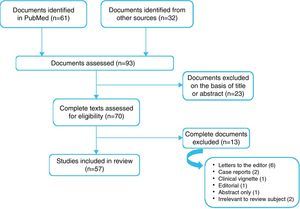
The filtered search yielded 61 articles, of which 23 were rejected on the basis of the title and abstract as being unrelated to the study aim. A complete reading was performed of the remaining 38, of which 13 were excluded from the present review: six letters to the editor, two case reports and one clinical vignette, one published only as an abstract, two that did not in fact refer to paradoxical AS, and one editorial that was considered irrelevant. In order not to miss any important articles, the reference lists of the selected articles were perused and 32 more publications were added to the original list. The guidelines on valvular disease of the American and European societies of cardiology were also consulted. The final list consisted of 57 articles (Figure 1).
The quality of the main studies analyzed in the present review was assessed (Supplementary Table 1) by an external consultant using the Effective Public Health Practice Project tool (http://www.ephpp.ca/tools.html).
ResultsThe search results produced 14 original articles on paradoxical AS, of which four were retrospective (6,8,54,55) and seven were prospective (11,16,17,22,41,52,53). Two substudies of randomized trials (49,51) and one of a multicenter registry (56) were also included.
The aim of around a third of the studies was to compare paradoxical AS with the classical AS phenotype (6,8,11,22,51). One study included a comparison with patients with moderate AS (8) and another with patients with LF-LG-AS (52). Two assessed the interaction between transvalvular gradient and SVI in patients with AVA of ≤1 cm2 and LVEF of ≤50% (16,17). Six focused on surgical treatment (6,8,16,17,22,53) and three on percutaneous treatment of patients with paradoxical AS (49,55,56). These 14 articles are summarized in Table 1.
Studies on paradoxical aortic stenosis and their main conclusions.
| Study and year | Type of study and sample | Main conclusions |
|---|---|---|
| Hachicha et al., 20078 | Retrospective study of 512 consecutive patients with severe AS (AVA <0.6 cm2/m2) and LVEF >50%, divided into two groups: -low flow: SVI ≤35 ml/m2 (n=181) – paradoxical AS -normal flow: SVI >35 ml/m2 (n=335) – classical AS | 1. 35% of patients presented SVI ≤35 ml/m2. 2. Patients with low-flow AS were mainly female, had small left ventricle with concentric remodeling and lower LVEF. Zva was higher. 3. SVI ≤35 ml/m2 was associated with lower three-year survival (76% vs. 86%, p<0.01). 4. Patients with low flow treated medically had worse outcome than those treated surgically at three years (58% vs. 93%, p=0.001). |
| Lee et al., 201113 | Prospective comparative study of 103 patients with AS and LVEF >50%, divided into two groups: -87 patients with normal flow AS; -16 patients with paradoxical AS (SVI ≤35 ml/m2). | 1. Patients with paradoxical AS had larger BSA, less hypertension and were more likely to have AF. 2. Patients with paradoxical AS were more symptomatic (p=0.02). 3. LVEF was significantly lower in the group with paradoxical AS. 4. Patients with paradoxical AS had greater myocardial thickness and were more likely to have greater concentric LV remodeling. 5. AVA and AVAi were significantly lower in the group with paradoxical AS. 6. GLS was significantly lower and Zva was significantly higher in the group with paradoxical AS (p<0.01 for both). 7. GLS showed a negative correlation with SVI (r=-0.324, p=0.001) and AVAi (r=-0.377, p<0.001) and a positive correlation with Zva (r=0.437, p<0.001). 8. Only age and Zva were significant determinants of GLS. 9. Patients with paradoxical AS had more cardiovascular events than those with normal-flow AS (93.8% vs. 69.8%). 10. Subclinical myocardial dysfunction may be more prominent in paradoxical AS compared with normal-flow AS. |
| Jander et al., 201152 | Prospective study of 1525 randomized patients in the SEAS trial, with asymptomatic AS and LVEF ≤55%. | 1. Severe AS was present in 29% of the population and moderate AS in 12%; 2% had severe AS with high gradient, 1% had AVA >1.0 cm2 and high gradient and 56% had AS with AVA >1.5 cm2. 2. Patients with low gradient were older, with lower BSA, more often female and had higher systolic BP, than those with moderate AS. 3. Patients with severe AS and low gradient had lower MPG and smaller AVA than those with moderate AS. 4. At one year, few aortic valve events occurred in patients with either severe low-flow AS or moderate AS (3.2% vs. 3.8%, p=0.71). 5. There were no differences in major cardiovascular events (4.8% vs. 4.3%, p=0.80) or cardiac death (1.1% vs. 0.5%, p=0.49) at one year between the group with severe low-gradient AS and the group with moderate AS. 6. Over the whole study period (45.8±14.1 months) aortic valve events occurred in 48.5% of patients with severe AS and low gradient vs. 44.6% in those with moderate AS. 7. Patients with severe LF-LG-AS (SVI ≤35 ml/m2, n=223) had similar rates of aortic valve events to those with normal-flow severe AS (46.2% vs. 50.9%, p=0.53). 8. In patients with severe low-gradient AS, cardiovascular mortality was similar in those undergoing SAVR and those treated medically (6.0% vs. 9.1%, p=0.23). 9. Patients with severe low-gradient AS had similar mortality from cardiovascular cause to those with classical severe AS (7.8% vs. 5.7%, p=0.65). 10. Progression to classical severe AS was seen in 38% of patients with moderate AS and 41% of those with severe low-gradient AS. 11. Patients with classical severe AS had more aortic valve events and major cardiovascular events than those with severe low-gradient AS or those with moderate AS (both p<0.01). |
| Clavel et al., 201210 | Retrospective study of 1589 patients with moderate AS and LVEF >50%, divided into three matched groups: -187 patients with severe paradoxical AS (AVA <1 cm2/m2; MPG <40 mmHg, SVI ≤35 ml/m2, LVEF ≤50%) (group 1) -187 patients with severe AS (AVA ≤0.6 cm2/m2; MPG >40 mmHg) (group 2) -187 patients with moderate AS (AVA >0.6 cm2/m2) (group 3) | 1. Patients with paradoxical AS were older, more often female, and had greater prevalence of CAD and hypertension, higher Zva and HR, and lower BSA, LVEF and LVEDV. 2. Patients with paradoxical AS had higher all-cause and cardiovascular mortality at one and five years than those with moderate or severe AS. 3. Aortic valve replacement predicted survival in patients with paradoxical and severe AS, but not in patients with moderate AS. 4. Factors independently associated with higher all-cause mortality were advanced age (p<0.01), conservative treatment (p<0.01), reduced LVEF (p=0.03) and paradoxical AS (p=0.02). 5. Prognosis of patients with paradoxical AS was worse than of patients with severe high-gradient or moderate AS. |
| Mohty et al., 201317 | Observational study of 768 patients with severe AS (AVA ≤1 cm2) and LVEF >50%, divided into four groups by MPG (<40 vs. ≥40 mmHg) and SVI (<35 vs. ≥35 ml/m2): -normal flow, high gradient (NFHG group); -low flow, low gradient (LFLG group); -low flow, high gradient (LFHG group); -low flow, low gradient (NFLG group). | 1. In the study population, 58% were male, 89% symptomatic, 50% hypertensive and 90.7% underwent SAVR. 2. The NFHG group accounted for 50% of the population, the LFLG group 13%, the LFHG group 15% and the NFLG group 22%. 3. Patients in the LFLG group were significantly older (p<0.01), with higher rates of AF (p<0.01), higher HR (p<0.01), lower LVEF (p=0.01) and a trend for higher rates of CAD (p=0.07) than in the other groups. 4. The LFLG group had significantly higher PVR and Zva (both p<0.01) than the other groups, but reduced SAC (p<0.01). 5. SAVR was less often performed in the LFLG group than in the other groups: 84% vs. 94% (NFHG), 87% (NFLG) and 91% (LFHG) (p<0.01). 6. Operative mortality in SAVR was higher in the LFLG group (p=0.01). 7. Survival at 10 years was lower in the LFLG group (32%) than in the NFHG group (66%), which had a more favorable 10-year prognosis. 8. After adjustment for other risk factors, LF-LG-AS was independently associated with lower long-term survival (p<0.01). 9. Patients in the LFLG group had better long-term survival when treated by SAVR than by medical therapy (p<0.01). 10. SAVR was significantly associated with better survival in the LFLG group (p<0.01) |
| O'Sullivan et al., 201356 | Retrospective study of 354 patients with severe AS (AVAi ≤0.6 cm2/m2 or MPG >40 mmHg) treated by TAVR, divided into three groups: -208 patients with MPG >40 mmHg (group 1); -85 patients with AVAi ≤0.6 cm2/m2, MPG <40 mmHg, SVI ≤35 ml/m2 and LVEF ≤50% (group 2); -61 patients with AVAi ≤0.6 cm2/m2, MPG ≤40 mmHg and LVEF ≤40% (group 3). | 1. Patients in group 2 had significantly greater AVAi than the other groups. 2. Compared to group 1, groups 2 and 3 had higher PVR (p<0.01) but lower Zva (p=0.027). 3. All-cause and cardiovascular mortality at 30 days differed between the three groups. 4. Functional improvement measured by NYHA class at one year was seen in most patients in all groups (p=0.09). 5. No significant differences in one-year mortality were seen between the three groups (p=0.67). |
Eleid et al., 201353 | Prospective comparative study of 24 patients with severe low-gradient (<40 mmHg) symptomatic AS (AVA ≤1 cm2 or AVAi ≤0.6 cm2/m2), divided into two groups: -18 patients with preserved LVEF (>50%) (group 1); -six patients with reduced LVEF (≤50%) (group 2). | 1. Patients in group 1 were older and most were hypertensive. 2. In group 1, all patients had pulmonary hypertension, elevated ventricular filling pressures and reduced SVI. 2. Patient in group 1 presented a close correlation between systolic aortic pressure and LV end-diastolic pressure (r=0.64, p<0.01). 3. No significant correlation was found in group 1 between systolic aortic pressure and MPG (r=-0.18, p=0.29). 4. All measures of LV afterload improved after nitroprusside infusion in both groups. 5. Nitroprusside infusion reduced mean aortic pressure, LV end-diastolic pressure and mean pulmonary artery pressure (p<0.05). 6. In group 1, MPG increased from 27 to 29 mmHg (p=0.02) after nitroprusside infusion and AVA increased from 0.86 to 1.02 cm2 (p<0.01). |
| Herrmann et al., 201350 | Multicenter randomized clinical trial (PARTNER) of 971 patients with severe AS (AVA <0.8 cm2 or AVAi <0.5 cm2/m2 and MPG ≤40 mmHg or PFV ≤4.0 m/s), divided into two cohorts: -Cohort A: high-risk patients; -Cohort B: inoperable patients. | 1. 55% of patients had low flow and 45% had normal flow. 2. 45% of patients had low gradient (≤40 mmHg). 3. 23% of patients had low flow and reduced LVEF (<50%). 4. 15% of patients had low flow, reduced LVEF (<50%) and MPG <40 mmHg. 5. The prevalence of paradoxical AS was 31%. 6. Patients with low flow had more comorbidities including CAD, pacemakers, symptoms of heart failure, and higher pulmonary artery pressures than patients with normal flow. 7. All-cause mortality at two years was significantly higher in patients with low flow compared to those with normal flow (47% vs. 34%, p<0.01). 8. Surgical or percutaneous valve replacement was associated with a marked increase in survival at one and two years: mortality with medical therapy was 76% at two years, compared to 38-46% with TAVR or SAVR. 9. Two-year survival was significantly better with SAVR than with medical therapy in cohort B (p<0.01). 10. No significant difference was seen in two-year survival in cohort A between SAVR and TAVR (p=0.47). 11. Patients in cohort A with low flow had higher mortality at two years than those with normal flow, whether treated by SAVR (38% vs. 29%) or by TAVR (40% vs. 25%). 12. Patients in cohort B with low flow had higher mortality than those with normal flow, but both improved with SAVR (46% vs. 76% with low flow and 38% vs. 53% with normal flow, p<0.01). 13. In patients with paradoxical AS mortality was lower following SAVR than with medical therapy (p<0.01). 14. In patients with paradoxical AS, SAVR reduced one-year mortality from 66% to 35% (p=0.02). 15. In patients with paradoxical AS in cohort A there was no significant difference between TAVR and SAVR (39% vs. 38.3%, p=0.69). 16. Only low flow was an independent predictor of mortality in both cohorts, while LVEF and MPG did not show a significant correlation. |
| Ozkan et al., 201354 | Prospective study of 260 patients with severe symptomatic AS (AVAi ≤0.6 cm2/m2), with MPG <40 mmHg and LVEF ≤50%. | 1. 53% of patients received medical therapy and 47% underwent TAVR or SAVR. 2. Compared to patients undergoing SAVR or TAVR, patients receiving medical therapy had a greater prevalence of diabetes, lower BP, greater use of diuretics and higher creatinine levels. 3. Patients undergoing TAVR were older and more often female than those undergoing SAVR. 4. Compared to patients with normal flow and low gradient, patients with low flow and low gradient had lower LVEF (62% vs. 59%, p<0.01), more severe AS (AVAi 0.46 vs. 0.35 cm2/m2, p<0.01) and higher Zva (3.9 vs. 5.6 mmHg/ml/m2, p<0.01). 5. During follow-up of 28±24 months, 105 patients died (40%): 30% of those undergoing SAVR and 70% of those treated medically. 6. SAVR was associated with more favorable outcome (p<0.01). 7. SAVR was independently associated with outcome (p<0.013). 8. Patients treated medically had two-fold higher all-cause mortality. 9. Patients with low flow and low gradient had similar survival after SAVR to those with normal flow and low gradient. 10. In unadjusted Kaplan-Meier analysis, patients with low flow and low gradient had worse outcome (p=0.007). 11. After adjustment for age, gender and treatment, there was no association between flow pattern and clinical outcome (p=0.64). |
| Eleid et al., 20137 | Observational study of 1704 consecutive patients with severe AS (AVA <1.0 cm2) and preserved LVEF (≤50%) assessed by two-dimensional echocardiography, divided into four groups according to SVI (<35 ml/m2 vs. ≤35 ml/m2) and MPG (<40 mmHg vs. ≤40 mmHg): -50 patients with low flow and high gradient (LF/HG); -53 patients with low flow and low gradient (LF/LG); -352 patients with normal flow and low gradient (NF/LG); -1249 patients with normal flow and high gradient (NF/HG). | 1. The NF/LG group (21%) had better survival with medical therapy (82% vs. 67% in the NF/HG group, p<0.01). 2. The LF/LG group (3%) had smaller LV size, higher Zva, lower LVEF, greater prevalence of AF and heart failure, and lower SAC and worse survival (60% vs. 82% in the NF/HG group, p<0.01). 3. Patients with AF were older, with higher resting HR and more symptoms. 4. Two-year survival was 60% in the LF/LG group compared to 85% in the NF/LG group, 82% in the NF/HG group and 78% in the LF/HG group (p<0.01). 5. Patients in the LF/LG group had higher all-cause mortality than in the other groups. 6. The low flow, low gradient pattern was the strongest predictor of mortality (p<0.01). 7. SAVR was associated with lower mortality of 69% in the LF/LG and NF/HG groups (p<0.01) but not in the NF/LG and LF/HG groups. |
| Mohty et al., 201455 | Retrospective study of 677 patients with severe AS (AVA ≤1 cm2) with preserved LVEF (≥50%) treated by SAVR. | 1. The prevalence of paradoxical AS was 26%. 2. After SAVR, 54% of patients had PPM (AVAi ≤0.85 cm2/m2). 3. Of patients with LF-LG-AS, 56% had PPM. 4. Patients with LF-LG-AS and PPM were significantly older and had more comorbidities than those without LF-LG-AS or PPM. 5. Ten-year survival was significantly lower in patients with LF-LG-AS and PPM than those without LF-LG-AS or PPM (38% vs. 70%, p=0.002). 6. The combination of LF-LG-AS before surgery and PPM after surgery was associated with worse outcome. |
| Clavel et al., 201441 | Cohort study of 400 patients with moderate to severe AS, divided into two cohorts: -Cohort A: 250 consecutive patients with paradoxical AS and severe AS with normal flow and high gradient (NF/HG), treated by SAVR; -Cohort B: 150 non-consecutive patients with moderate to severe AS, preserved LVEF and normal flow, treated by SAVR. | 1. Patients with paradoxical AS had a greater prevalence of dyslipidemia and CAD, lower SVI, smaller LV chambers, lower MPG and higher Zva, compared to patients with NF/HG in cohort A. 2. The weight of excised valves was less and prevalence of bicuspid phenotype was lower in patients with paradoxical AS (15% vs. 42% in NF/HG patients). 3. Applying the cut-offs for weight of excised valves to indicate severity (≤2.0 g for males and ≤1.2 g for females), 70% of patients with paradoxical AS and 86% of patients with NF/HG had severe AS. |
| Maes et al., 201422 | Prospective study of 349 patients with severe AS and preserved LVEF, divided into two groups according to MPG: -high gradient (n=144), MPG >40 mmHg; -LF-LG-AS (n=205), MPG ≤40 mmHg. | 1. Patients in the two groups had similar clinical and demographic characteristics. 2. AF and diabetes were more prevalent in patients with paradoxical AS. 3. Patients with paradoxical AS had greater AVAi and ELCo, lower LVEDD and LVEDV, lower SVI and greater Zva, compared to patients with high-gradient AS. 4. All-cause survival at four years was higher in patients with paradoxical AS than in patients with high-gradient AS. 5. MPG was not significantly associated with four-year survival in patients with severe AS. 6. Patients with paradoxical AS were less often referred for SAVR than those with high-gradient AS (7% vs. 17%, p<0.01). 7. Survival without SAVR was better in patients with paradoxical AS than in those with high-gradient AS in the entire study population (p<0.01) and in symptomatic patients (NYHA class I or II) (p=0.002). 8. The variables independently associated with clinical outcome were age, LV volume, presence of COPD and diabetes, and NYHA class. 9. In symptomatic patients (NYHA class I or II), factors independently associated with clinical outcome were MPG, age, diabetes and LV volume. 10.SAVR significantly improved survival in both groups. 11. The protective effect of SAVR tended to be stronger in patients with high-gradient AS than in those with paradoxical AS, since the latter had better clinical outcomes when not operated. 12. 82% of patients with paradoxical AS evolved over time to a more severe form of AS, with increased MPG, and half of these developed severe high-gradient AS. 13. In patients with high-gradient AS, the minority who evolved to a low-gradient form developed the classical form of LF-LG-SA. 14. Survival of patients with paradoxical AS was better after SAVR than with medical therapy. |
| Lauten et al., 2014 57 | Prospective study of 3077 consecutive patients from the GARY (German Aortic Valve Registry) registry treated by TAVR, divided into three groups: -group 1: severe LF-LG-SA (LVEF ≤40%, MPG <40 mmHg, and AVAi ≤0.6 cm2/m2); -group 2: paradoxical LF-LG-AS (LVEF ≥50%, MPG <40 mmHg and AVAi ≤0.6 cm2/m2); -group 3: severe high-gradient AS (MPG ≤40 mmHg and AVAi ≤0.6 cm2/m2). | 1. Group 1 represented 11.7% of the study population, group 2 (paradoxical AS) 20.8% and group 3 60.6%. 2. Patients with paradoxical AS were slightly younger than those in group 3 (p<0.01) but older than those in group 1 (p=0.001); there were more females in groups 2 and 3. 3. Group 1 presented more comorbidities than groups 2 and 3, in which comorbidities were similar. 4. Mortality during hospitalization for SAVR was higher in group 1 than in group 3 (p=0.029) and similar between groups 2 and 3 (p=0.67). 5. At one year, there was a more marked difference between mortality in group 1 and mortality in the other two groups (32.3% vs. 22.3% in group 2, p=0.01, and 19.8% in group 3, p<0.001). 6. There was no significant difference in mortality between groups 2 and 3 one year after SAVR (p=0.192). 7. The rate of major cardiac and cerebral events at one year after SAVR was higher in group 1 than in the other two groups (group 1: 34.5% vs. group 2: 27.5%, p=0.021, and group 3: 23.8%, p<0.001). 8. Independent predictors of in-hospital mortality were 3-vessel CAD, pulmonary hypertension and peripheral vascular disease. 9. Specifically for patients with paradoxical AS, independent predictors of in-hospital mortality were 3-vessel CAD (p=0.018) and COPD (p=0.005). |
AF: atrial fibrillation; AS: aortic stenosis; AVA: aortic valve area; BP: blood pressure; BSA: body surface area; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; ELCo: energy loss coefficient; GLS: left ventricular global longitudinal strain; HR: heart rate; AVAi: indexed aortic valve area; LF-LG-AS: low-flow, low-gradient aortic stenosis; LV: left ventricular; LVEDD: left ventricular end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; MPG: mean pressure gradient between aorta and left ventricle; NYHA: New York Heart Association; PFV: peak flow velocity; PPM: patient-prosthesis mismatch; PVR: peripheral vascular resistance; SAC: systemic arterial compliance; SAVR: surgical aortic valve replacement; SVI: stroke volume index; TAVR: transcatheter aortic valve replacement; Zva: valvuloarterial impedance.
Paradoxical AS is defined as AVA of ≤1 cm2, MPG of <40 mmHg, and SVI of ≤35 ml/m2, despite LVEF of ≤50% (Figure 1).8 This condition is usually the result of marked LV concentric remodeling with interstitial myocardial fibrosis, and reduction of the ventricular chamber and its compliance.8,11–15 There is reduced longitudinal myocardial strain in the phenotype of paradoxical AS despite good global LV systolic function.6
Low systolic flow hampers assessment of the severity of AS, since the gradient is proportional to the fourth power of the flow, and any reduction in flow leads to a large reduction in gradient and hence underestimation of disease severity.9 Furthermore, transvalvular flow rate depends not only on ejection volume but also on ejection time.19 Other factors such as atrial fibrillation (AF), mitral regurgitation, mitral stenosis and tricuspid regurgitation can also play an important part in reducing ejection volume and thus contribute to situations of low flow and low gradient.7,16
Clinical characteristicsEstimates of the prevalence of paradoxical AS range between 3% and 35% of patients with severe degenerative AS (Figure 2).6,7 This discrepancy may be due to the characteristics of study populations.8,16 For example, in an echocardiographic study of 1704 consecutive patients with severe AS and preserved LVEF (≤50%), Eleid et al.7 found a prevalence of paradoxical AS of only 3%, whereas in another echocardiographic study, of 397 patients with AS, Kusunose et al.6 documented a prevalence of paradoxical AS of 35%. Another study, on a sample of patients with severe AS referred for cardiac catheterization,17 observed a prevalence of 26%, a similar figure to echocardiographic studies.
Paradoxical AS appears to be more frequent in females and older patients. Compared to classical severe high-gradient AS, more patients with paradoxical AS have a history of hypertension, diabetes, AF and coronary artery disease.6,7,9,10,17,18 Hypertension is common in patients with AS, but blood pressure (BP) in those with paradoxical AS may be pseudonormalized by the low-flow pattern associated with increased vascular stiffness.8 Its symptomatology is similar to that of classical severe AS, although its onset is more insidious.1,10
Diagnosis of paradoxical aortic stenosisWhen paradoxical AS is suspected, as well as PFV, aortic transvalvular gradients and AVA, assessment should include vascular load, global LV hemodynamic load, LV function beyond LVEF, LV geometry, and myocardial damage.19
Transthoracic echocardiography is the method of choice for hemodynamic study in AS. Cardiac catheterization is not routinely indicated except when echocardiography is not diagnostic or when there are discrepancies in clinical findings.20,21
Problems with calculation of aortic valve areaAVA is calculated by the continuity equation, which is based on the principle of conservation of mass, assuming that the volume that passes through a given area upstream of the aortic valve is the same as that which passes through the valve. This measure is less flow-dependent than other parameters such as velocity and gradient, but is susceptible to error, since it is only an indirect measure of valve area. Firstly, the continuity equation measures the size of the functional orifice instead of that of the anatomic orifice and thus neglects the coefficient of orifice contraction, and so under normal flow conditions underestimation of the anatomic orifice by the continuity equation is expected to be around 10-15%.22 Furthermore, LV ejection volume (the numerator in the continuity equation) is usually calculated by two-dimensional echocardiography on the basis of cross-sectional area, assuming the left ventricular outflow tract (LVOT) to be circular. However, Doddamani et al. used multislice computed tomography (CT)23 and three-dimensional echocardiography24 to show that the LVOT is in fact more often elliptical than circular. This can result in AVA being underestimated by a further 15%.24
Since the severity of AS can be under- or overestimated if the patient has a large or small body surface area (BSA), respectively, it is essential to index valve area to BSA, yielding AVAi, although it should be noted that such indexing may not be appropriate at extreme values of BSA.1
Consistency between echocardiographic criteria of AS severity (AVA by the continuity equation, PFV and MPG) was established following an analysis of 3482 echocardiograms of patients with good global LV systolic function and AVA <2 cm2. It was shown that the criterion of AVA ≤1 cm2 overestimated severity compared to the other two criteria (PFV ≤4 m/s and MPG ≤40 mmHg). The authors accordingly suggested that the cutoff of AVA to define severe AS should be <0.8 cm2, which more often corresponds to PFV of ≤4 m/s and MPG of ≤40 mmHg. A limitation was that the authors did not present the clinical results of this reclassification.25
To summarize, although AVA is an essential parameter in the stratification of AS severity, there are significant limitations to its measurement.
Three-dimensional echocardiography is an alternative method to measuring AVA that has been shown to be more accurate in assessing AVA and AS severity than volumetric methods such as the continuity equation by two-dimensional echocardiography.26
Projected AVA (AVAproj) is another way to estimate AVA in situations of low systolic flow and associated general systolic dysfunction. It is based on a normal systolic flow (250 ml/ms) by the formula
where VC is vascular compliance and Qrest is systolic ejection flow at rest. AVAproj, which is obtained during dobutamine stress echocardiography, appears to offer a good alternative to standard echocardiographic parameters.27Assessment of vascular componentsVascular alterations are particularly important in paradoxical AS. Peripheral BP should be assessed and systemic arterial compliance and peripheral vascular resistance should also be estimated.19 In a patient with reduced systolic flow, it is important to perform echocardiographic assessment under conditions of controlled BP.19,28
Valvuloarterial impedance (Zva) is a simple and non-invasive measure of the double hemodynamic load (vascular and valvular) on the left ventricle. It is calculated by dividing LV systolic pressure (the sum of systolic BP and mean transvalvular gradient) by indexed stroke volume.29,30 This parameter has been shown to have prognostic value29 and is a good marker of myocardial dysfunction.31 In a substudy of the SEAS (Simvastatin Ezetimibe in Aortic Stenosis) study,32 Zva was the main predictor of ventricular dysfunction in patients with severe AS. In a retrospective study, Hachicha et al.29 identified Zva of >4.5 mmHg/ml/m2 as indicating severely increased LV afterload, which was associated with 2.8-fold and 3.7-fold increases in all-cause and cardiovascular mortality, respectively. The same study showed that Zva was particularly high in paradoxical AS,29 and it was subsequently confirmed that patients with paradoxical AS present higher Zva than those with classical severe AS.10 Nevertheless, Zva was not shown to be a good predictor of long-term mortality following valve surgery.30
Quantification of intrinsic left ventricular functionAortic valve replacement is a class I recommendation for patients with severe AS and LV dysfunction as assessed by LVEF, independently of associated symptoms, in the European and American guidelines on valvular disease.1,3 Although widely used, LVEF is prone to measurement errors and is not considered an accurate indicator of LV contractility.19 There is thus a need for a more precise measure of LV function, using other parameters, in patients with paradoxical AS.19
Global longitudinal strain (GLS) is a complementary measure of myocardial function, assessed by speckle-tracking echocardiography.33 It appears to be less influenced by LV geometry and is considered superior to LVEF for assessment and quantification of intrinsic myocardial function.19 Several studies have shown that GLS is an early marker of LV dysfunction that decreases gradually with progressively more severe AS.19 In the study by Lancellotti et al.,34 GLS of ≤15.9% was associated with higher rates of cardiovascular events in patients with AS. The segmental heterogeneity of GLS in AS has been demonstrated,35 with reduced GLS more evident in basal segments. However, the use of global and regional strain parameters in paradoxical AS is the subject of debate, and there is disagreement on their value for diagnosis, risk stratification and prognosis. Nevertheless, in the study by Kusunose et al.,6 GLS was an independent predictor of mortality (hazard ratio 1.05; 95% confidence interval 1.03-1.07; p<0.01), a cutoff of -12.1% being associated with significantly higher mortality. The study by Sato et al.36 came to the same conclusion, although with a cutoff of -17% to predict cardiovascular events. These authors suggest that assessment of GLS could have an important role in risk stratification of patients with paradoxical AS.
Assessment of myocardial damageProlonged stress on myocardial fibers leads to cellular damage and death by apoptosis. Interstitial myocardial fibrosis is therefore a common finding on cardiac magnetic resonance imaging and histological studies,19 and is associated with persistence of LV dysfunction even after surgical aortic valve replacement (SAVR).37
Readily available biomarkers such as brain natriuretic peptide (BNP) and N-terminal pro-brain natriuretic peptide (NT-proBNP) reflect LV systolic and diastolic dysfunction, and the more they are elevated, the more severe the symptoms and the worse the prognosis.37,38 It should be noted that these peptides do not measure AS severity directly, but only myocardial function, and are not specific to AS.19,38
New parameters for assessment of paradoxical aortic stenosisNew parameters for assessment of AS have appeared that are improving understanding of the disease.
The degree of aortic valve calcification is a strong predictor of disease progression.19 Although it can be semi-quantitatively assessed by echocardiography, a more precise quantification of calcification is provided by CT.19 According to the American guidelines, a calcium score of >1000 Agatston units identifies severe aortic valve calcification.3 This method is not affected by the patient's hemodynamic characteristics and is thus useful in low-flow states such as paradoxical AS.39 Messika-Zeitoun et al.40 demonstrated a strong association between aortic valve calcification and AVA (r=-0.79, p<0.01).
In a study of 250 consecutive patients treated by SAVR, Clavel et al.41 found that in 70% of patients with paradoxical AS, stenosis was considered severe on the basis of the weights of valves excised during surgery. This percentage is similar to that found in patients with classical severe high-gradient AS (86%).41 This study confirms that a large proportion of patients with paradoxical AS in fact have a severe form of the disease and highlights the limitations of the echocardiographic methods used for stratifying AS patients, especially valve gradients.41
Current guidelines do not differentiate between methods for measuring AVA (catheterization or Doppler echocardiography). AVA can be assessed invasively using the Gorlin formula,20 but this is dependent on systolic flow and is unsuitable in low cardiac output states. Doppler echocardiography measures the pressure gradient through the valve based on peak systolic flow at the vena contracta, while catheterization determines the pressure gradient between the left ventricle and the ascending aorta, several cm from the valve.19 The total energy of aortic flow is the sum of kinetic and static energy (pressure). Flow convergence through the stenotic valve converts static to kinetic energy, reducing pressure at the vena contracta. When velocity falls distal to the stenosis, part of the energy is converted back to static energy due to the phenomenon of pressure recovery.42 The clinical implication of this phenomenon is that Doppler echocardiography underestimates AVA compared to invasive assessment.28 To overcome this limitation, Garcia et al. developed a new index, the energy loss coefficient (ELCo),43 to adjust AVA to pressure recovery in the ascending aorta.28 ELCo is calculated by the formula
where AA is the cross-sectional area of the ascending aorta, measured 1 cm downstream of the sinotubular junction.19,43 This index has a good association with prognosis in patients with severe AS, particularly for values ≤0.6 cm2/m2.44 It is also useful in paradoxical AS, giving values close to those from invasive assessment and appearing to have prognostic value.28 Some authors suggest it should be used routinely to assess AS severity, particularly when the diameter of the thoracic aorta is small (<30 mm).42As mentioned above, AS is nowadays considered to be a systemic vascular disease, with increased systemic vascular stiffness. Recent studies have shown that the vascular mechanics of the aorta can be assessed by speckle-tracking echocardiography, an imaging indicator of vascular stiffness,45,46 and that values are lower in AS than in aortic regurgitation.45 Moreover, patients with moderate to severe low-flow AS present lower aortic strain values than those with normal systolic flow.46 Although promising, assessment of valve mechanics by speckle-tracking echocardiography is currently only a research tool due to limitations arising from the need for a good acoustic window on the aorta and the lengthy analysis required.
New markers, including neurohormones, adipokines and extracellular matrix modulators, have recently been identified as prognostic and diagnostic factors of severe low-flow, high-gradient AS with preserved LVEF.47 Their future potential for assessment of patients with paradoxical AS should be the subject of further research.
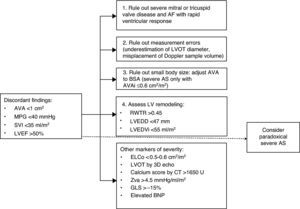
DiagnosisWhen assessment of a patient with AS reveals discordant values of AVA and MPG (AVA ≤1 cm2 and MPG <40 mmHg), and there is good LV systolic function but reduced systolic flow (SVI ≤35 ml/m2), the possibility of paradoxical AS should be considered. According to Pibarot et al.,48 a systematic approach is required (Figure 3). Other morbidities such as significant mitral or tricuspid valve disease should be ruled out at the outset, as should measurement errors and misplacement of the Doppler sample volume. Echocardiographic assessment should be performed under normal blood pressure conditions. AVA should be indexed to BSA and body mass index should also be considered. An AVAi of ≤0.6 cm2/m2 indicates severe AS. Signs of LV remodeling suggest the presence of paradoxical severe AS. When the severity of AS is in doubt, additional information can be obtained from other parameters such as aortic valve calcium score, anatomical AVA, Zva, ELCo, and strain parameters, which may indicate not only severity but also the diagnosis of paradoxical AS.19
Algorithm for diagnosis of paradoxical aortic stenosis. 3D echo: three-dimensional echocardiography; AF: atrial fibrillation; AS: aortic stenosis; AVA: aortic valve area; AVAi: aortic valve area indexed to body surface area; BSA: body surface area; BNP: brain natriuretic peptide; CT: computed tomography; ELCo: energy loss coefficient; GLS: global longitudinal strain; LV: left ventricular; LVEDD: left ventricular end-diastolic diameter; LVEDVi: indexed left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; MPG: mean pressure gradient between aorta and left ventricle; RWTR: relative wall thickness ratio; SVI: stroke volume index; Zva: valvuloarterial impedance.
Various studies have indicated that the combination of low systolic flow, low transvalvular gradient, and preserved global systolic function is a marker of poor outcome.
- •
Hachica et al.,8 in a retrospective study of 512 consecutive patients with severe AS and preserved LVEF, showed that low flow (SVI ≤35 ml/m2) was associated with worse outcome at three years.
- •
Mohty et al.,17 in a study of 768 patients with severe AS and preserved LVEF, observed that low flow and low gradient was associated with reduced long-term survival after adjustment for other factors.
- •
In an observational study of 1704 consecutive patients with severe AS and preserved LVEF, Eleid et al.7 showed that the low-flow, low-gradient phenotype was the strongest predictor of mortality.
- •
Lancellotti et al.,49 in a prospective study of 150 consecutive patients with severe AS, demonstrated that patients with low flow and low gradient had worse clinical outcomes than those with normal flow and low gradient; in this study both low flow and low gradient were independent predictors of poor outcome.
- •
In a subanalysis of 971 patients with severe AS of the Placement of AoRTic TraNscathetER Valve Trial Edwards SAPIEN Transcatheter Heart Valve (PARTNER) trial, Herrmann et al.50 showed that only low flow independently predicted mortality in both cohort A (high-risk patients) and cohort B (inoperable patients). All-cause mortality at two years was also significantly higher in low-flow than in normal-flow AS.
There is thus general agreement in the literature that low-flow states in severe AS with good global systolic function are associated with worse medium- and long-term prognosis.10 Cardiovascular events are more frequent in patients with paradoxical AS than in those with normal-flow AS,13,51 and survival at one and five years in a retrospective study of 1589 patients by Clavel et al. was less than in patients with moderate AS.10
Not all authors draw the same conclusions. In a recent study,22 Maes et al. prospectively assessed 349 patients with severe AS and preserved LVEF, divided into two groups according to MPG (>40 mmHg or ≤40 mmHg). Prognosis as measured by all-cause survival at four years was better in patients with paradoxical AS than in those with classical severe high-gradient AS.
Similarly, in a subanalysis of the SEAS study, Jander et al.52 showed that patients with paradoxical AS had similar clinical outcomes to those with moderate AS.
Natural historyThe natural history of paradoxical AS has been the subject of intense debate since 2007, and as reported above, there seems to have been a change in the disease paradigm. As shown in Model A (Figure 4), after a stage in which AS reaches a certain level of severity, in the absence of treatment the patient may evolve to a phenotype in which LV dilatation and systolic dysfunction predominate, leading to reduced systolic flow and transvalvular gradients. The initial studies by Hachicha et al.8 and Lancellotti et al.49 suggested that patients with severe AS can also evolve to a different phenotype. In this view, paradoxical AS represents an advanced stage of aortic valve disease due to marked LV interstitial fibrosis, reduced chamber size and increased systemic vascular stiffness, leading to diminished LV systolic flow and hence reductions in pressure gradients and aortic valve area. As described above, the fact that patients with paradoxical AS have worse outcome than those with classical (high-gradient) severe AS corroborates this model.
Recent findings, however, point to a different evolution of the disease (Model B in Figure 4). Maes et al.22 suggest that paradoxical AS represents an intermediate stage in progression to severe high-gradient AS. The prognosis of patients with paradoxical AS is similar to that of those with moderate AS and is better than in high-gradient severe forms. Furthermore, in the absence of valve replacement, severe AS evolves to a low-flow, low-gradient form and not to paradoxical AS. According to Maes et al., the concept of paradoxical AS is probably due to inaccuracies in the calculation of AVA using the continuity equation, as discussed above.
TreatmentAS should not be considered an isolated disease of the aortic valve, but rather a systemic vascular disease involving atherosclerosis and arteriosclerosis. In addition, its clinical manifestations also depend on ventricular remodeling and LV function.8 Treatment should bear all these factors in mind.8
Vascular componentRegarding the vascular component of AS, from a clinical standpoint it is important to optimize BP.8,53 Antihypertensive therapy reduces afterload and LV filling pressures as well as pulmonary artery pressure.53 No specific antihypertensive drug class is recommended for AS in the American and European guidelines1,3 However, diuretics should be used with care in patients with paradoxical AS to avoid excessive reductions in preload, which can further reduce systolic ejection volume. Vasodilators are not apparently contraindicated in these patients, but this is based on a single study in an acute setting using intravenous sodium nitroprusside. In a prospective study of 24 patients with paradoxical AS, Eleid et al.53 demonstrated that sodium nitroprusside infusion reduced total LV afterload with no significant secondary effects.
Valvular componentAs pointed out above, paradoxical severe AS may represent a more advance stage of the disease and therefore have a worse prognosis.8,41,50 In the presence of typical symptoms and the absence of contraindications, patients should therefore undergo SAVR.8,17,41,50,54 In the American and European guidelines, SAVR is a class IIa recommendation for patients with confirmed severe LF-LG-AS and preserved LVEF.1,3 However, patients with paradoxical AS are in fact less often referred for surgery,8,10,17,28 both because of underestimation of AS severity by the standard assessment parameters (AVA, MPG and SVI) and due to the epidemiological context of the disease.10,17,41
Surgical aortic valve replacementIn patients with paradoxical AS, SAVR is demonstrably superior to medical therapy in improving prognosis, as confirmed by various authors.
- •
Hachicha et al.,8 in their retrospective study of 512 consecutive patients with severe AS and preserved LVEF, observed that patients with low-flow AS treated medically had worse three-year survival than those treated surgically (58% vs. 93%, p<0.01).
- •
In the retrospective study of 1589 patients with moderate or severe AS and LVEF >50% by Clavel et al.,10 SAVR was an independent predictor of survival in the paradoxical AS group (Figure 5).
Figure 5.Kaplan-Meier analysis of combined (aortic valve replacement [AVR], or death) events (A), AVR (B), overall survival (C), and cardiovascular survival (D). In (A), event-free survival at one and five years was 63% and 24% for paradoxical AS, 30% and 9% for severe high-gradient AS, and 85% and 41% for moderate AS, respectively (p<0.01). In (B), it can be seen that patients with paradoxical AS were less often referred for AVR than those with severe high-gradient AS but more often than those with moderate AS.10 Overall and cardiovascular survival (C and D, respectively) were lower in paradoxical AS than in high-gradient severe AS and moderate AS. HG-SAS group: patients with high-gradient severe aortic stenosis; MAS group: patients with moderate aortic stenosis; PLG-SAS group: patients with paradoxical low-flow, low-gradient, severe aortic stenosis.
Figure reproduced from Clavel et al.10 with permission from Elsevier.
- •
Mohty et al.,17 in their observational study of 768 patients with severe AS and LVEF >50% divided into four groups according to MPG (<40 vs. ≥40 mmHg) and SVI (<35 vs. ≥35 ml/m2), reported that patients with LF-LG-AS who underwent SAVR had better long-term survival than those treated medically.
- •
In their prospective study of 260 patients with severe symptomatic AS with MPG <40 mmHg and LVEF ≤50%, Ozkan et al.54 demonstrated that SAVR was associated with better outcome and that mortality in patients treated medically was twice that in patients undergoing SAVR.
- •
The observational study by Eleid et al.7 of 1704 consecutive patients with severe AS and preserved LVEF, divided into four groups according to SVI and MPG, showed that SAVR was associated with lower mortality in patients with low flow and low gradient.
By contrast, Maes et al.,22 in their prospective study of 349 patients with severe AS and preserved LVEF, demonstrated that survival without SAVR was better in patients with paradoxical AS compared to those with classical high-gradient severe AS. In this study, low flow did not influence prognosis. However, all groups showed better survival after SAVR. The same study showed that survival of patients with paradoxical AS was better after SAVR compared to medical therapy.22
It should be noted that operative mortality in patients with paradoxical AS is higher than in those with severe high-grade AS,17 probably due to older age, more comorbidities (both cardiovascular and non-cardiovascular) and, as explained above, possibly a more advanced stage of the disease.17 Furthermore, patients with paradoxical AS are prone to patient-prosthesis mismatch (PPM) (AVAi ≤0.85 cm2/m2) and the combination of paradoxical AS and postoperative PPM is associated with worse prognosis.55
Transcatheter aortic valve replacementIn patients considered unsuitable for SAVR due to contraindications or high surgical risk, transcatheter aortic valve replacement (TAVR) is an alternative treatment.1 TAVR leads to better outcomes and long-term survival than medical therapy, and its results in paradoxical AS are similar to those in classical severe AS, as shown by the following studies.
- •
O'Sullivan et al.,56 in a retrospective study of 354 patients with severe AS treated by TAVR, showed that patients with paradoxical AS had similar mortality to those with classical severe AS.
- •
In a subanalysis of the PARTNER trial of 971 randomized patients with severe AS, Herrmann et al.50 demonstrated that in paradoxical AS, TAVR reduced mortality from 73% to 43% in inoperable patients (p<0.01) compared to medical therapy.
- •
In their prospective study of 3077 consecutive patients from the GARY (German Aortic Valve Registry) registry treated by TAVR, Lauten et al.57 showed that clinical outcomes were similar between paradoxical AS and classical severe AS and that there was no significant difference between the groups in terms of mortality at one year after the procedure.
The decision whether to proceed with TAVR in patients with paradoxical AS depends on the heart team, who review indications and contraindications for valve replacement locally and on a case-by-case basis.1 Another subanalysis of the PARTNER trial found no significant differences in mortality among high-risk patients between SAVR and TAVR (39% vs. 38.3%, p=0.69), and the debate continues concerning the best treatment for patients with paradoxical AS.49
To summarize, the literature seems relatively unanimous on the need for valve replacement, whether surgical or percutaneous, in symptomatic patients with paradoxical AS.1 As shown above, both options improve prognosis, survival and clinical status in patients with paradoxical AS compared to medical therapy. The final decision concerning which treatment to adopt will depend on assessment of the patient's surgical risk and the logistics of the center involved.1
DiscussionParadoxical AS was described for the first time in 2007 and its phenotypic characteristics have been confirmed by various groups. However, questions remain concerning inconsistencies in the continuity equation for measurement of AVA that may explain the conflicting findings in this phenotype and could lead to reclassification of some forms of severe AS as moderate AS. Non-invasive diagnostic methods have been developed that can better identify the paradoxical form of severe AS, including assessment of LV remodeling and of the vascular component, measurement of VA and of the LVOT by three-dimensional methods (tomographic and non-tomographic), and calculation of the calcium score of the aortic valve.
The data presented above appear to indicate that the prognosis of patients with paradoxical AS is poor, although it is not clear whether model A or B (Figure 4) explains the stage it represents in the natural history of AS.
It should be noted that most of the studies analyzed indicate that the prognosis of patients with paradoxical AS is better following valve replacement than with medical therapy. In the European and American guidelines valve replacement is a class IIa recommendation (albeit with level of evidence C) for symptomatic paradoxical AS. The limitations of studies assessing the prognostic impact of valve replacement in patients with paradoxical AS – including patient selection, study design (single center, retrospective analysis, lack of randomization) and confounding variables – are summarized in Supplementary Table 1.
ConclusionsThe phenotype of paradoxical AS, characterized by low systolic ejection volume and low gradient, is common in patients with AS and preserved LV systolic function. Measurement errors, inconsistencies in the continuity equation and lack of BP control can lead to contradictory findings when assessing a patient with AS. Although there is some disagreement in the literature, the prognosis of symptomatic paradoxical AS is poor and in such cases valve replacement should be considered.
LimitationsThe present review includes only articles published up to December 2014. It should be noted that the representativeness of the studies in the quality assessment was low (25%).
ContributionsRC performed the bibliographic search, produced the literature summary and wrote the first version of the manuscript. RT designed the review, discussed the results, modified the initial document for submission and responded to the reviewers. MJV carried out the quality assessment and played an important part in the response to the reviewers. LG contributed to the discussion of the results and to correction of the manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cavaca R, Teixeira R, Vieira MJ, Gonçalves L. Estenose aórtica paradoxal – revisão sistemática. Rev Port Cardiol. 2017;36:287–305.








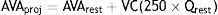
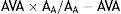
 AVA: aortic valve area;
AVA: aortic valve area; 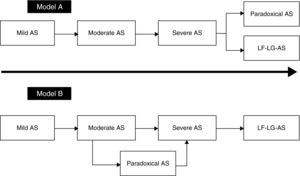 LF-LG-AS: low-flow, low-gradient aortic stenosis.'/>
LF-LG-AS: low-flow, low-gradient aortic stenosis.'/>![Kaplan-Meier analysis of combined (aortic valve replacement [AVR], or death) events (A), AVR (B), overall survival (C), and cardiovascular survival (D). In (A), event-free survival at one and five years was 63% and 24% for paradoxical AS, 30% and 9% for severe high-gradient AS, and 85% and 41% for moderate AS, respectively (p<0.01). In (B), it can be seen that patients with paradoxical AS were less often referred for AVR than those with severe high-gradient AS but more often than those with moderate AS.10 Overall and cardiovascular survival (C and D, respectively) were lower in paradoxical AS than in high-gradient severe AS and moderate AS. HG-SAS group: patients with high-gradient severe aortic stenosis; MAS group: patients with moderate aortic stenosis; PLG-SAS group: patients with paradoxical low-flow, low-gradient, severe aortic stenosis. Figure reproduced from Clavel et al.10 with permission from Elsevier.](https://static.elsevier.es/multimedia/21742049/0000003600000004/v1_201704230025/S2174204917300545/v1_201704230025/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)



