Tricuspid stenosis (TS) is an uncommon complication of transvenous ventricular pacemaker implantation, with few cases reported in the literature.
The mechanisms described are obstruction of right ventricular inflow by tricuspid vegetations (endocarditis), multiple pacemaker leads and tricuspid valve (TV) fibrosis secondary to perforation or laceration of the TV leaflets, or adherence between redundant portions of the lead and valvular and subvalvular tissue.
We report two cases of severe TS, with different etiologies and management: one caused by leaflet perforation, resolved surgically, and the other secondary to fusion between a loop of the pacemaker lead and the subvalvular apparatus, which was treated medically.
A estenose tricúspide (ET) é uma complicação rara da implantação transvenosa de pacemakeres ventriculares, com poucos casos relatados na literatura. Os mecanismos descritos são a obstrução do fluxo ao nível da válvula tricúspide (VT) por vegetações (endocardite), por múltiplos elétrodos ou por fibrose do tecido valvular ou subvalvular secundária a perfuração ou laceração dos folhetos VT ou como resultado da aderência de porções redundantes do eletrocateter aos folhetos e tecido subvalvular. Relatamos 2 casos de ET severa, com etiologia e abordagem terapêutica diferente. O primeiro causado pela perfuração do folheto valvular pelo eletrocateter, resolvido cirurgicamente e o outro, como consequência da adesão de uma ansa formada pelo eletrocateter e o aparelho subvalvular tratado medicamente.
We present the case of a 62-year-old man implanted with a permanent VVIR pacemaker in 1993 following admission for symptomatic complete atrioventricular block. In 1997 the generator was replaced due to inflammation and necrosis of the pacemaker pocket, with no signs of infection or endocarditis. In 2002 he was rehospitalized with the same clinical setting; the generator implanted on the right was removed, keeping the ventricular lead, and a new system was implanted on the left, programmed in DDDR mode. In October 2007, he went to the emergency department with exertional dyspnea (NYHA class III/IV), CCS class II angina, lower limb edema, asthenia and anorexia, of three months’ evolution. Physical examination revealed jugular vein distension, grade II/VI systolic murmur and low-frequency diastolic murmur, hepatomegaly and lower limb edema extending to the upper thigh. The electrocardiogram showed atrial fibrillation alternating with ventricular pacemaker rhythm. Laboratory tests showed elevated gamma-glutamyltransferase (GGT), alkaline phosphatase and BNP.
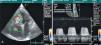
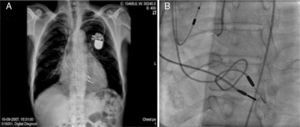
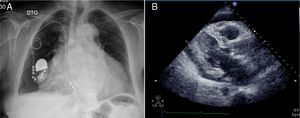
The chest X-ray revealed cardiomegaly and a redundant loop of one of the ventricular leads (Figure 1A). Transthoracic echocardiography showed a moderate circumferential pericardial effusion, severe right atrial dilatation and tricuspid valve (TV) fibrosis with stenosis (functional area 0.6cm2, peak and mean gradient of 16mmHg and 13mmHg, respectively), and mild regurgitation, with no other significant alterations (Figure 2A and B). Transesophageal echocardiography was performed to characterize TV morphology, which revealed marked thickening and reduced mobility of the leaflets and subvalvular apparatus, severely limiting valve opening (mean gradient 12mmHg), and mild regurgitation. Cardiac catheterization showed decreased TV opening, with a mean gradient of 8mmHg and grade II/IV tricuspid regurgitation, with normal pulmonary artery (34mmHg) and right ventricular pressures (Figure 1B). Coronary angiography revealed significant disease of the proximal right coronary artery, but no other significant alterations.
The patient was transferred to a cardiothoracic surgery center, where he underwent tricuspid valvotomy (commissurotomy between the anteroseptal and posteroseptal leaflets) and saphenous vein bypass graft to the right coronary artery. Severe right atrial dilatation was observed during surgery, together with marked TV deformation, with extremely reduced opening and pronounced fibrosis along the course of the leads, one of which had perforated the septal leaflet. The endocardial pacemaker was replaced by an epicardial system. The remainder of his hospital stay was uneventful and 15 months after the surgery the patient is asymptomatic, the echocardiogram showing TS (mean gradient 3mmHg) and moderate regurgitation.
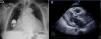
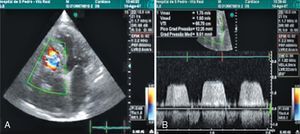
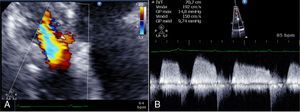
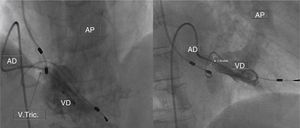
Case 2We present the case of an 80-year-old woman implanted with a permanent VDD pacemaker in 2001 for symptomatic conduction tissue disease, who underwent generator replacement due to battery depletion in February 2009. In August 2010 she went to the emergency department with a clinical setting of exertional dyspnea (NYHA class III/IV), orthopnea, edema, predominantly right quadrant abdominal pain, asthenia and anorexia, progressively worsening over the previous two months. Physical examination showed jugular vein distension, facial cyanosis, generalized edema, hepatomegaly and rapid irregular peripheral pulse; pulmonary auscultation revealed no signs of congestion, while cardiac auscultation revealed soft heart sounds and an aortic mid-systolic murmur and a low-frequency diastolic murmur at the left sternal border. The electrocardiogram showed atrial fibrillation with controlled ventricular rate alternating with ventricular pacemaker rhythm. The chest X-ray revealed cardiomegaly, obliteration of the costophrenic angles and a redundant loop of the ventricular lead at the level of the tricuspid valve apparatus, with no other significant abnormalities (Figure 3A). Laboratory tests detected slight elevation of BNP and GGT but no other relevant alterations. Echocardiography revealed thickened TV leaflets and the ventricular lead adhering to the valve and subvalvular apparatus, severely limiting opening (peak and mean gradient of 15 and 10mmHg, respectively, and estimated functional area of 0.6cm2) (Figure 4), aortic sclerosis and a moderate pericardial effusion at the left ventricular free wall (1.6cm) and large effusion at the right ventricular wall (2.4–2.1cm), with slight right ventricular diastolic collapse (Figure 3B). Right heart catheterization showed malpositioning of the pacemaker lead at the level of the TV, which was deformed, with reduced and eccentric opening (mean gradient of 11mmHg) (Figure 5). Left heart catheterization revealed a deformed and calcified aortic valve (maximum ventricular–aortic gradient of 13mmHg with preserved left ventricular function – ejection fraction 62%), and coronary atherosclerosis with wall calcification but no evidence of significant stenosis.
(A) Chest X-ray showing cardiomegaly, obliteration of the costophrenic angles and a redundant loop of the ventricular lead at the level of the tricuspid valve apparatus; (B) B-mode echocardiogram showing moderate pericardial effusion at the left ventricular free wall (1.6cm) and large effusion at the right ventricular wall (2.4–2.1cm).
The patient was transferred to a cardiothoracic surgery center, where it was decided to perform pericardiocentesis and to optimize medical therapy. In view of symptomatic improvement, a conservative approach was adopted, with subsequent reassessment of the need for surgical intervention. Six months after discharge, she is in NYHA functional class II/IV, echocardiography showing severe TS and mild aortic stenosis, and no pericardial effusion.
DiscussionTricuspid stenosis is an uncommon complication of transvenous pacemaker implantation, with few cases reported in the literature. The mechanisms described are right ventricular inflow obstruction by tricuspid vegetations (endocarditis) or multiple pacemaker leads and fibrosis secondary to mechanical trauma.1–4 Trauma associated with endocardial systems may be due to perforation or laceration of the TV leaflets, or adherence between redundant loops and valve tissue. The resulting endothelial damage triggers a series of local events, including chronic inflammation, fibrosis, calcification and in some cases valve stenosis. A similar process has been described in an anatomopathological study of patients with implantable cardioverter-defibrillators.5 There have been three cases reported of TS secondary to fibrosis resulting from leaflet perforation, and four caused by adherence of redundant loops of the lead. In most of these cases, TS was associated with more than one lead crossing the TV; the time between initial pacemaker implantation and development of symptoms ranged between seven and 33 years.2 The therapeutic approach was medical management in three cases, valve replacement in two, surgical valvuloplasty in one and balloon angioplasty in one.4 In the first case presented here, TS was caused by leaflet perforation, diagnosed 14 years after pacemaker implantation, and was successfully treated by surgical valvuloplasty. In the second case, TS was the result of adherence of a redundant loop to the tricuspid valve apparatus, which was diagnosed nine years after implantation, and was treated medically.
ConclusionsLead-induced TS is a chronic complication of pacemaker implantation that, while rarely reported, may occur more often than it is suspected clinically, and could become even more common in the future due to the growing number of cardiac devices implanted and their long-term use. The diagnosis should be considered in any patient with endocardial leads and signs or symptoms of right heart failure. The therapeutic approach should be decided on a case-by-case basis in consultation with a multidisciplinary team, including the cardiologist and the surgeon, and taking into consideration the patient's wishes.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ribeiro, H; Estenose tricúspide induzida por electrocateter de pacemaker: relato de 2 casos clínicos. Rev Port Cardiol. 2012. doi:10.1016/j.repc.2012.02.008