We report the case of a 50-year-old woman with systemic lupus erythematosus who had previously undergone pacemaker implantation. She developed recurrent pulmonary thromboembolism and was diagnosed with antiphospholipid syndrome. During investigation, pacemaker endocarditis was discovered, and the system was surgically explanted. Surprisingly, all microbiological studies, including culture of the extracted material and extensive serological analysis, were negative and she remained well with anticoagulation plus her usual immunosuppressant regimen.
The data indicate that her pacemaker endocarditis could be an equivalent of the nonbacterial thrombotic endocarditis often described in native valves.
Descreve-se o caso de uma doente de 50 anos com lúpus eritematoso sistémico e história prévia de implantação de pacemaker. Esta desenvolveu tromboembolismo pulmonar recorrente, tendo-lhe sido diagnosticada síndrome antifosfolipídica. Durante a investigação foi descoberta uma endocardite de pacemaker, tendo sido sujeita a extracção cirúrgica do sistema. Inesperadamente, todos os exames microbiológicos, incluindo culturas do material removido, e extensa análise serológica, foram negativos, tendo a doente se mantido clinicamente estável com anticoagulação oral e o habitual regime imunossupressor.
Os dados indicam que a esta endocardite de pacemaker poderá ser um equivalente da endocardite trombótica não bacteriana, habitualmente descrita em válvulas nativas.
Infective endocarditis is a serious infection that can involve intracardiac devices and cause significant problems.1,2
The entity designated as nonbacterial thrombotic endocarditis (NBTE) is not associated with infection, and should be considered in the differential diagnosis of culture-negative endocarditis.3
We report an unusual case of pacemaker endocarditis in a patient with autoimmune disease, in the sense that the vegetations appeared to relate to the underlying autoimmune disease and not to infection.
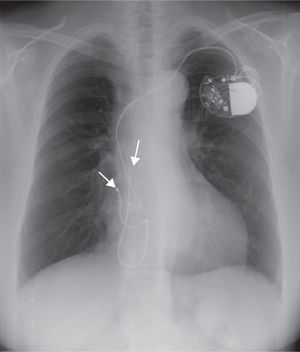
Case reportWe describe the case of a 50-year-old woman with a history of systemic lupus erythematosus (SLE) diagnosed in the second decade of life (1988), already with involvement of multiple organs and with previous hospital admissions because of disease complications, including lupus cerebritis and avascular necrosis of the femoral head. She was later diagnosed with symptomatic sinus dysfunction and underwent pacemaker implantation (DDDR mode) by the right subclavian route (1997). Three years after implantation there were signs of subcutaneous erosion in the pacemaker pocket and a surgical revision was performed with placement of a new generator. Three months after this procedure, a pacemaker pocket infection was detected and the system was explanted, except the atrial lead, which was sectioned and abandoned in the atrial cavity. A new system (AAIR mode) was implanted on the contralateral side (Figure 1). In the following eight years no pacemaker-related events occurred. She was admitted to the hospital with pulmonary thromboembolism in 2008. Her previous follow-up records showed that she had had several episodes of autoimmune disease exacerbation in recent years, with acute serositis and sicca syndrome, and was currently medicated with corticosteroids, hydroxychloroquine and azathioprine. She began oral anticoagulation with warfarin and was discharged uneventfully. In the following year she had three additional hospital admissions for recurrent segmental pulmonary embolism (diagnosed by CT angiography), although her INR was in the therapeutic range. All studies (including transthoracic echocardiograms) failed to show any embolic focus, but serological studies were positive for antiphospholipid antibodies. She was considered to have antiphospholipid syndrome associated with LES, which could explain the recurrent thromboembolism. The oral anticoagulation dosage was increased and she remained free of new events in the following year. In the meantime, a new pacemaker generator was placed because of battery depletion, without complications. In July 2010, during a routine follow-up in the autoimmune clinic, she complained of pain and swelling in the right arm. She had elevation of inflammatory markers and Doppler echocardiography showed decreased pulsatility in the right brachiocephalic trunk, which was suggestive of subclavian vein thrombosis. Inflammatory marker levels continued to rise in the following weeks, with all microbiological exams negative and normal scintigraphy with marked leukocytes. As part of the study, a new transthoracic echocardiogram was performed that showed two masses in the right atrium adherent to a pacemaker lead, with a high degree of motility, one of them prolapsing into the right ventricle during diastole (Figure 2). A diagnosis of pacemaker endocarditis was made and the pacemaker system was extracted surgically and an epicardial pacemaker system was implanted. The histopathological analysis of the masses (Figure 3) was compatible with vegetations. Nevertheless, cultures of the removed material were negative for bacteria and all previous blood cultures were negative, with incubation periods exceeding two weeks. She was started on an antibiotic regimen after the surgery, but this was rapidly suspended because of the negative microbiological results. New blood cultures were collected at different intervals, including some specific for fungi, that were also negative. All the serological tests, including those specific for viruses, Brucella, Bartonella and Coxiella, were also negative. On the basis of these findings, it was proposed that she had NBTE associated with her autoimmune disease and not a bacterial infection. The patient maintained her usual immunosuppressant drugs and oral anticoagulation, and no additional course of antibiotics was prescribed. She remained clinically well, with no further events, with decreasing levels of inflammatory markers and normal postoperative transthoracic and transesophageal echocardiograms.
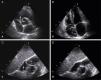
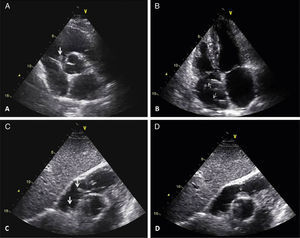
Transthoracic echocardiographic images. A – Parasternal short-axis view showing a mass prolapsing into the right ventricle (large arrow). B – Apical 4-chamber view demonstrating a pacemaker lead in the right atrium (small arrows). C – Modified subcostal view showing two mobile masses adherent to a pacemaker lead (large arrows). D – One of the masses is shown prolapsing into the right ventricle (small arrow).
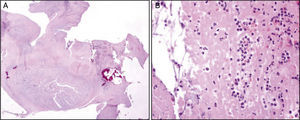
Pathological findings. A – Low amplification view of a section of one of the masses described above. B – High amplification view shows it is composed essentially of fibrin, infiltrated by inflammatory cells, which was found to be compatible with a vegetation, but with no discernible bacteria.
The latest data show that, even after extensive study, 7% of pacemaker endocarditis cases are culture-negative.2 It has been demonstrated that nonbacterial thrombotic endocarditis is a differential diagnosis to be considered in culture-negative endocarditis.1 In this entity there are vegetations not related with bacterial infections, but with immune complex deposition and subsequent inflammatory reaction. It has been associated with numerous conditions, including cancer and autoimmune diseases, particularly antiphospholipid syndrome.3,4
In our case, we confirmed the presence of masses with a histopathology pattern compatible with vegetations, but microbiological studies, including several blood cultures taken on different occasions and culture of the surgically extracted material, were all negative. Additionally, all serological tests for viruses and specific bacteria were negative. It is also significant that the patient remained clinically well, with declining levels of inflammatory markers, maintaining her usual immunosuppressant drugs and oral anticoagulation, without antibiotics. Finally, it is important to note that the patient suffers from SLE with antiphospholipid syndrome, conditions that have a close association with NBTE. So, based on the above data, we postulated that this pacemaker endocarditis must be an equivalent of the nonbacterial thrombotic endocarditis usually described in native valves, associated with her autoimmune disease.
Reviewing the literature on this subject, we found that this entity mainly affects the left-sided valves.3,4 Nevertheless, NBTE has been reported in the right atrium and right-sided valves, although very rarely.5
The recurrent pulmonary thromboembolism that the patient suffered could probably be explained by embolization of part of the vegetations that in initial phases could be too small to be detected by a transthoracic echocardiogram. In fact, it is frequently stated in the literature that the vegetations associated with NBTE are especially friable and prone to embolization.4,5 Considering this hypothesis, the absence of pulmonary infection or abscess is further evidence that supports the sterility of the vegetations.
Funding sourcesNo funding sources were used for the study.
Conflicts of interestThe authors have no conflicts of interest to declare.