Percutaneous coronary intervention (PCI) with paclitaxel drug-eluting balloons (DEBs) is used mainly for treatment of in-stent restenosis (ISR) and small vessel disease. Our objective was to evaluate the clinical efficacy of this strategy in a multicenter registry.
MethodsBetween 2009 and 2010 a prospective registry from two centers enrolled 156 consecutive patients undergoing PCI with at least one DEB. A primary composite endpoint of major adverse cardiac events (MACE) (all-cause death, myocardial infarction [MI] and target lesion revascularization [TLR]) was assessed at one year follow-up. Stepwise Cox regression was used to determine independent predictors of outcome.
ResultsDEBs (n=206) were used to treat 184 lesions. Procedural success was obtained in 98% of patients (n=150). At one-year follow-up, 86% (n=134) were free of the primary endpoint (6% death, 6% non-procedure related MI and 5% TLR). The independent predictors of MACE at one year were index PCI in the left anterior descending artery (HR 2.81, 95% CI 1.21–6.51; p=0.02) and a history of MI (HR 3.46, 95% CI 1.35–8.84; p=0.01). ISR and DEB diameter or length were not predictors of events.
ConclusionsPCI with DEBs in real-world patients with complex lesions is effective, with a low rate of MACE, including TLR, at one-year follow-up. The results are equally good whether the intervention is for ISR or for native coronary disease.
A intervenção coronária percutânea (ICP) com balão eluidor de fármaco (DEB) tem vindo a ser utilizada no tratamento da reestenose intra-stent (RIS) e na doença coronária de pequenos vasos. O objectivo foi avaliar a eficácia clínica desta estratégia num registo multicêntrico.
MétodosRegisto prospetivo de dois centros com 156 doentes (dts) consecutivos incluídos, entre 2009 e 2010, submetidos a ICP com pelo menos um balão DEB. Definiu-se como endpoint primário a ocorrência combinada (MACE) de todas as causas de morte, EAM e revascularização da lesão alvo (TLR) a um ano de seguimento. Determinou-se os preditores independentes de prognóstico através da análise de regressão de Cox.
ResultadosForam tratadas 184 lesões com 206 DEB. O sucesso do procedimento foi obtido em 98% (150 dts). A um ano de seguimento, a sobrevida livre de endpoint composto ocorreu em 134 dts e foi de 86% (morte em 6%, EAM em 6% e TLR em 5%). Os preditores independentes de MACE foram a ICP na artéria descendente anterior (HR 2,81, 95% IC 1,21-6,51, p = 0,02) e história prévia de EAM (HR 3,46, 95% IC 1,35-8,84, p = 0,01). O diâmetro ou comprimento do DEB e a RIS não foram preditores de eventos.
ConclusõesA ICP com DEB em dts do mundo real e neste cenário complexo de lesões, é eficaz com baixa taxa de MACE a um ano de seguimento, incluindo TLR. Os resultados são igualmente bons se a intervenção é no contexto de RIS ou na doença coronária de novo.
Balloon angioplasty offered a valid alternative to surgery for coronary revascularization,1 but the risk of acute coronary occlusion and high restenosis rates associated with recoil, negative remodeling and cell proliferation have fueled an ongoing search for new therapeutic techniques that carry less risk of restenosis.2
The development of drug-eluting stents (DES) considerably reduced the risk of restenosis by eliminating recoil and negative remodeling and by significantly suppressing neointimal hyperplasia.3 Nevertheless, concerns about the risk of stent thrombosis, dependence on prolonged dual antiplatelet therapy and the risk of restenosis in patients with complex lesions prompted the search for alternative devices with low restenosis risk but without the disadvantages associated with DES,4 which led to the development of drug-eluting balloons (DEB).2
Several randomized studies demonstrated the safety and efficacy of this new technology for the treatment of in-stent restenosis, which was the first indication approved for their use in coronary artery disease (CAD).5,6 Other studies subsequently widened indications to other areas of endovascular intervention, notably de novo small vessel disease and peripheral arterial disease.4
It is thus important to compare the good results obtained in clinical trials with those in real-world patients through prospective multicenter registries of all-comers populations. The objective of this study was to evaluate the clinical efficacy of percutaneous coronary intervention (PCI) with DEBs in a multicenter national registry.
MethodsPopulationThis was a prospective study of consecutive patients undergoing PCI with DEBs in two Portuguese tertiary interventional cardiology centers. The registry included 220 patients, but only those who underwent PCI with DEB more than 12 months previously were assessed in the study in order to ensure at least one-year follow-up. Patients aged over 18 and with clinical evidence of myocardial ischemia were eligible for the study; there were no exclusion criteria in terms of CAD presentation or lesion complexity.
Data were collected prospectively and recorded in a database (Cardiobase®, Infortucano™) used by both interventional cardiology units. All patients gave their informed consent.
Percutaneous coronary interventionThe techniques employed in the PCI procedures were in accordance with generally accepted good practice. The selection of technique and revascularization strategy was at the discretion of the operators, as was choice of vascular access, use of glycoprotein IIb/IIIa inhibitors, and need for post-dilatation or bail-out stenting. Administration of an intravenous bolus of 70–100 U/kg unfractionated heparin was recommended.
DEBs coated with paclitaxel (3 μg/mm2 balloon surface) were used (SeQuent® Please, B-Braun™), with diameters ranging between 2.5 mm and 4.0 mm and lengths between 10 mm and 30 mm. An artery/balloon ratio of 1:1 and inflation time of 60 s were recommended.
All patients were medicated with clopidogrel (75 mg in those under chronic treatment [>10 days], and 600 mg otherwise) at the time of the intervention. After PCI, all patients were medicated with aspirin (≤150 mg/day) and clopidogrel (75 mg/day) for a minimum of three months, followed by single antiplatelet therapy.
Follow-up and study endpointFollow-up was by telephone or by consultation of the patients’ medical records. All patients were assessed at 30 days and followed for at least one year or until their death.
The study's composite endpoint – major adverse cardiac events (MACE) (all-cause death, non-fatal myocardial infarction [MI] and target lesion revascularization [TLR]) – was assessed at one year.
Deaths were classified as cardiac unless there was an unequivocal non-cardiac cause, in which case they were classified as non-cardiac. MI was defined according to the universal definition.7 TLR included percutaneous or surgical reintervention ≤5 mm from the proximal or distal edge of a previously treated segment.
Definite and probable stent thrombosis were defined according to the Academic Research Consortium criteria.8
Device success was defined as successful crossing of the lesion and expansion of the balloon; angiographic success was defined as final TIMI flow 3, with ≤30% residual stenosis; and procedural success was defined as device success with no occurrence of MACE during the index hospitalization.
Statistical analysisThe Kolmogorov–Smirnov test was used to test all continuous variables for normal distribution and Levene's test was used for homogeneity of variance. Continuous variables were presented as means ± standard deviation and compared using the Student's t test; variables that did not show normal distribution or homogeneity of variance were presented as medians and interquartile range (IQR) and compared using the Mann–Whitney test. Categorical variables were presented as percentages and compared by the chi-square test or Fisher's exact test, as appropriate.
Hazard ratios (HR) and 95% confidence intervals (CI) were calculated for occurrence of MACE at one-year follow-up. Cox regression analysis was performed to assess the impact of demographic, clinical and procedural characteristics on the study's endpoint at one year. Variables were selected for multivariate analysis according to their weight in univariate analysis (p<0.1 and <95% CI). The final model was constructed using forward stepwise selection with p=0.05 for entry and p=0.1 for exit. The cumulative incidence of MACE was calculated according to the Kaplan–Meier method and differences assessed by the log-rank test.
Values of p<0.05 were considered statistically significant. The statistical analysis was performed using SPSS, version 17.0 (Chicago, IL, USA).
ResultsBaseline characteristicsThe study population consisted of 156 consecutive patients (mean age 66±10 years, 73% male) who underwent PCI with paclitaxel-coated balloons. Their baseline characteristics and clinical presentation of the index PCI are shown in Table 1.
Baseline characteristics of the study population (n=156).
| Age, years ± SD | 66±10 |
| Male, n (%) | 114 (73) |
| Cardiovascular risk factors, n (%) | |
| Diabetes | 68 (44) |
| Hypertension | 129 (83) |
| Dyslipidemia | 120 (77) |
| Smoking | 78 (50) |
| Family history of CAD | 15 (10) |
| BMI, kg/m2 (±SD) | 26±7 |
| Previous CV history, n (%) | |
| MI | 71 (46) |
| PCI | 118 (76) |
| CABG | 32 (21) |
| Peripheral vascular disease | 16 (10) |
| Stroke | 10 (6) |
| Chronic renal failure, n (%) | 14 (9) |
| Left ventricular dysfunction, n (%) | 33 (21) |
| Multivessel CAD, n (%) | 111 (71) |
| Clinical presentation, n (%) | |
| Stable angina | 93 (60) |
| Unstable angina | 15 (10) |
| Non-ST elevation MI | 35 (22) |
| ST-elevation MI | 13 (8) |
BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CV: cardiovascular; MI: myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation.
This was a high-risk cohort, 44% with diabetes and 83% with hypertension. Most patients presented previously treated CAD (76% previous PCI and 21% coronary artery bypass grafting [CABG]). Around three-quarters of the population had two- or three-vessel disease and PCI was often performed in the context of MI (30%).
Procedure characteristicsIn the 156 patients, 184 lesions were treated with 206 DEBs (Table 2). The left anterior descending (LAD) was the most frequently affected artery (43%). Indications for use of DEBs were equally divided between in-stent restenosis in 52% (30% of which were bare metal stents [BMS] and 70% DES) and de novo CAD in 48%, mainly of small vessels. The pattern of in-stent restenosis was diffuse in 48% of cases. Most patients (80%) underwent pre-dilatation prior to use of DEBs. Median DEB diameter and length were 2.5 mm (IQR 2.5–3.0 mm) and 20 mm (IQR 15–26 mm), respectively. Five lesions were treated with bail-out stenting (four due to dissection and one due to significant recoil). Device success was 98%: it was not possible to cross the lesion in three patients, one with in-stent restenosis of a posterolateral artery (DEB 2.75 mm/15 mm) and two with small-vessel disease in the distal circumflex and mid LAD (DEB 2.5 mm/15 mm and 2.5 mm/17 mm, respectively). Angiographic success per lesion was 98% (two lesions with final TIMI flow 2 and two with >30% residual stenosis). Procedural success was 96%.
Procedure characteristics: 156 patients, 184 lesions.
| Target vessel, n (%) | |
| Left anterior descending | 67 (43) |
| Circumflex | 38 (24) |
| Right coronary | 39 (25) |
| Left main | 9 (6) |
| Bypass | 3 (2) |
| Classification of in-stent restenosis, n (%) | 81 (52) |
| I | 42 (52) |
| II | 17 (21) |
| III | 8 (10) |
| IV | 14 (17) |
| Type of stent with restenosis, n (%) | |
| BMS | 24 (15) |
| DES | 56 (36) |
| Unknown | 1 (0.6) |
| De novo coronary lesions, n (%) | 74 (48) |
| Small vessel disease | 64 (41) |
| Calcified lesions | 6 (4) |
| Following PCI with BMS | 4 (3) |
| Pre-dilatation, n (%) | 124 (80) |
| DEB characteristics, median (IQ) | |
| Length, mm | 20 (15–26) |
| Diameter, mm | 2.5 (2.5–3.0) |
| Inflation pressure, bar | 12 (10–14) |
| Inflation time, s | 60 (43–90) |
| Post-dilatation, n (%) | 4 (3) |
| Bail-out stent (per lesion), n (%) | 5 (3) |
| Device success (per lesion), n (%) | 181 (98) |
| Angiographic success (per lesion), n (%) | 180 (98) |
| Procedural success, n (%) | 150 (96) |
BMS: bare metal stent; DES: drug-eluting stent; DEB: drug-eluting balloon; IQ: interquartile range.
At 30-day follow-up there had been six MACE (two deaths from cardiac cause, four MI and two TLR).
At one-year follow-up nine patients (5.8%) had died (seven from cardiac cause), nine (5.8%) had suffered MI, and eight (5.1%) had undergone TLR, resulting in a combined incidence of events of 14.1% (Table 3). There were no documented cases of definite or probable stent thrombosis.
Adverse cardiovascular events.
| 30 days | 1 year | |
| TLR, n (%) | 2 (1.3) | 8 (5.1) |
| MI, n (%) | 4 (2.6) | 9 (5.8) |
| CV death, n (%) | 2 (1.3) | 7 (4.5) |
| Total deaths, n (%) | 2 (1.3) | 9 (5.8) |
| MACE, n (%) | 6 (3.8) | 22 (14.1) |
CV: cardiovascular; MACE: major adverse cardiac events (all-cause death, nonfatal myocardial infarction and target lesion revascularization); MI: myocardial infarction; TLR: target lesion revascularization.
There were no significant differences in rates of MACE or TLR between the groups treated for in-stent restenosis or for de novo CAD (13.6% vs. 14.7% MACE, p=0.846, and 6.2% vs. 4.0% TLR, p=0.721). One-year MACE and TLR rates were similar (14.3% vs. 12.5%; p=1.000, and 5.4% vs. 8.3%; p=0.633, respectively) in those treated for DES or BMS restenosis.
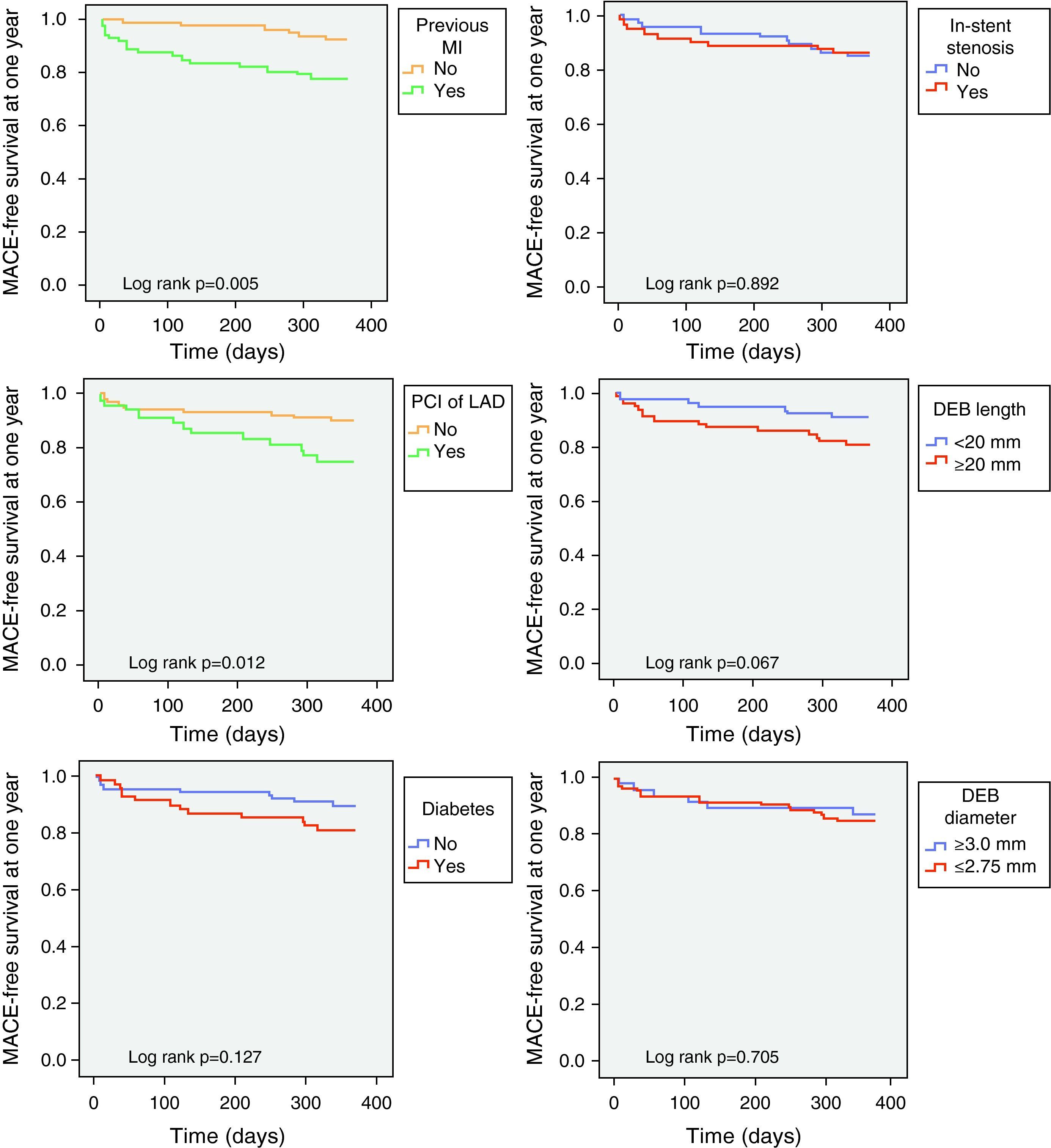
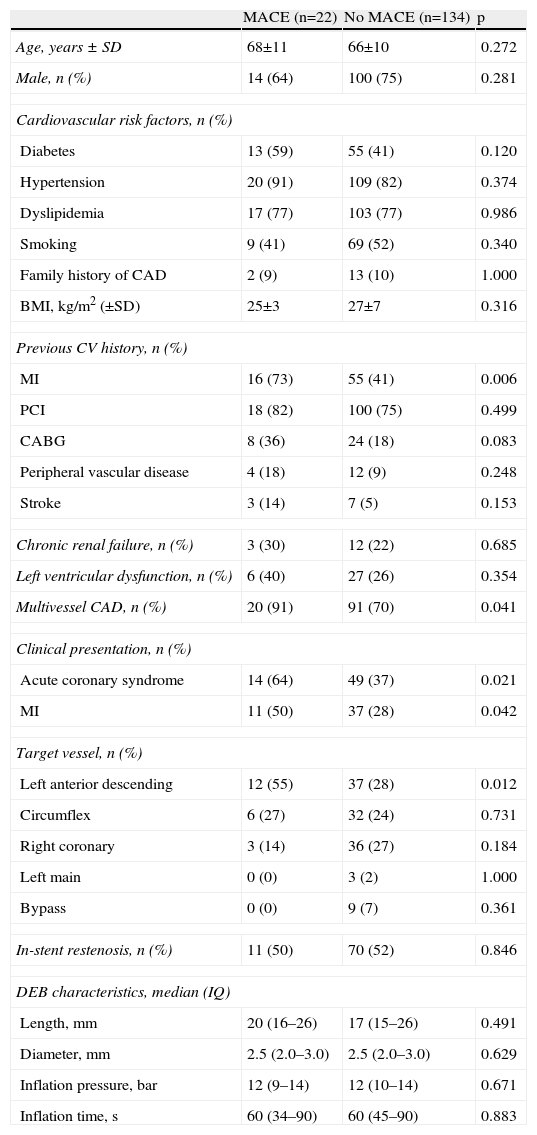
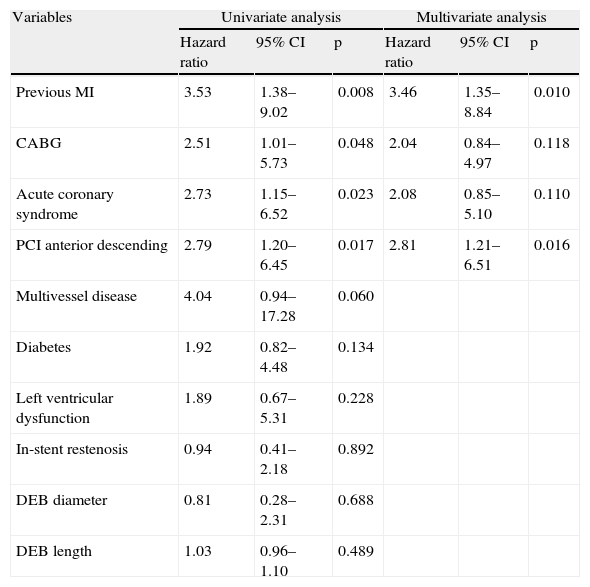
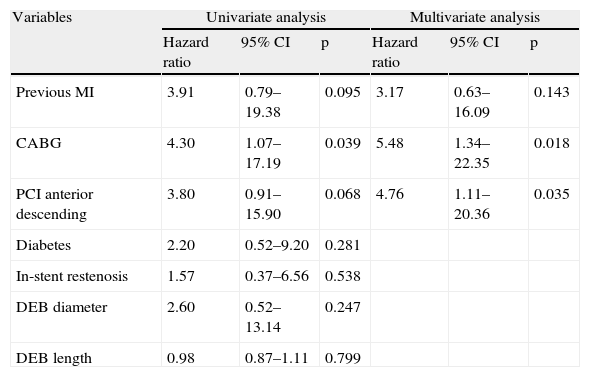
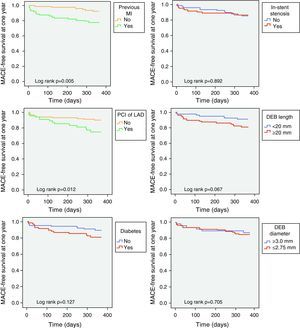
Predictors of MACE at one year in univariate analysis (Tables 4 and 5) were previous MI and CABG, acute coronary syndrome at presentation, and index PCI in the LAD; multivessel disease showed a trend for more MACE (HR 4.04; 95% CI 0.94–17.28; p=0.060). Previous CABG was the only predictor of TLR at one year (Table 6). Diabetes, left ventricular dysfunction, in-stent restenosis, and DEB diameter or length were not predictive of one-year MACE or TLR.
Univariate analysis of baseline and procedure characteristics according to major adverse cardiac events.
| MACE (n=22) | No MACE (n=134) | p | |
| Age, years ± SD | 68±11 | 66±10 | 0.272 |
| Male, n (%) | 14 (64) | 100 (75) | 0.281 |
| Cardiovascular risk factors, n (%) | |||
| Diabetes | 13 (59) | 55 (41) | 0.120 |
| Hypertension | 20 (91) | 109 (82) | 0.374 |
| Dyslipidemia | 17 (77) | 103 (77) | 0.986 |
| Smoking | 9 (41) | 69 (52) | 0.340 |
| Family history of CAD | 2 (9) | 13 (10) | 1.000 |
| BMI, kg/m2 (±SD) | 25±3 | 27±7 | 0.316 |
| Previous CV history, n (%) | |||
| MI | 16 (73) | 55 (41) | 0.006 |
| PCI | 18 (82) | 100 (75) | 0.499 |
| CABG | 8 (36) | 24 (18) | 0.083 |
| Peripheral vascular disease | 4 (18) | 12 (9) | 0.248 |
| Stroke | 3 (14) | 7 (5) | 0.153 |
| Chronic renal failure, n (%) | 3 (30) | 12 (22) | 0.685 |
| Left ventricular dysfunction, n (%) | 6 (40) | 27 (26) | 0.354 |
| Multivessel CAD, n (%) | 20 (91) | 91 (70) | 0.041 |
| Clinical presentation, n (%) | |||
| Acute coronary syndrome | 14 (64) | 49 (37) | 0.021 |
| MI | 11 (50) | 37 (28) | 0.042 |
| Target vessel, n (%) | |||
| Left anterior descending | 12 (55) | 37 (28) | 0.012 |
| Circumflex | 6 (27) | 32 (24) | 0.731 |
| Right coronary | 3 (14) | 36 (27) | 0.184 |
| Left main | 0 (0) | 3 (2) | 1.000 |
| Bypass | 0 (0) | 9 (7) | 0.361 |
| In-stent restenosis, n (%) | 11 (50) | 70 (52) | 0.846 |
| DEB characteristics, median (IQ) | |||
| Length, mm | 20 (16–26) | 17 (15–26) | 0.491 |
| Diameter, mm | 2.5 (2.0–3.0) | 2.5 (2.0–3.0) | 0.629 |
| Inflation pressure, bar | 12 (9–14) | 12 (10–14) | 0.671 |
| Inflation time, s | 60 (34–90) | 60 (45–90) | 0.883 |
BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CV: cardiovascular; DEB: drug-eluting balloon; IQ: interquartile range; MACE: major adverse cardiac event; MI: myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation.
Major adverse cardiac events at one year.
| Variables | Univariate analysis | Multivariate analysis | ||||
| Hazard ratio | 95% CI | p | Hazard ratio | 95% CI | p | |
| Previous MI | 3.53 | 1.38–9.02 | 0.008 | 3.46 | 1.35–8.84 | 0.010 |
| CABG | 2.51 | 1.01–5.73 | 0.048 | 2.04 | 0.84–4.97 | 0.118 |
| Acute coronary syndrome | 2.73 | 1.15–6.52 | 0.023 | 2.08 | 0.85–5.10 | 0.110 |
| PCI anterior descending | 2.79 | 1.20–6.45 | 0.017 | 2.81 | 1.21–6.51 | 0.016 |
| Multivessel disease | 4.04 | 0.94–17.28 | 0.060 | |||
| Diabetes | 1.92 | 0.82–4.48 | 0.134 | |||
| Left ventricular dysfunction | 1.89 | 0.67–5.31 | 0.228 | |||
| In-stent restenosis | 0.94 | 0.41–2.18 | 0.892 | |||
| DEB diameter | 0.81 | 0.28–2.31 | 0.688 | |||
| DEB length | 1.03 | 0.96–1.10 | 0.489 | |||
CABG: coronary artery bypass grafting; CI: confidence interval; DEB: drug-eluting balloon; MACE: major adverse cardiac events; MI: myocardial infarction; PCI: percutaneous coronary intervention.
Target lesion revascularization at one year.
| Variables | Univariate analysis | Multivariate analysis | ||||
| Hazard ratio | 95% CI | p | Hazard ratio | 95% CI | p | |
| Previous MI | 3.91 | 0.79–19.38 | 0.095 | 3.17 | 0.63–16.09 | 0.143 |
| CABG | 4.30 | 1.07–17.19 | 0.039 | 5.48 | 1.34–22.35 | 0.018 |
| PCI anterior descending | 3.80 | 0.91–15.90 | 0.068 | 4.76 | 1.11–20.36 | 0.035 |
| Diabetes | 2.20 | 0.52–9.20 | 0.281 | |||
| In-stent restenosis | 1.57 | 0.37–6.56 | 0.538 | |||
| DEB diameter | 2.60 | 0.52–13.14 | 0.247 | |||
| DEB length | 0.98 | 0.87–1.11 | 0.799 | |||
CABG: coronary artery bypass grafting; CI: confidence interval; DEB: drug-eluting balloon; MACE: major adverse cardiac events; MI: myocardial infarction; PCI: percutaneous coronary intervention.
After adjustment for demographic and baseline clinical characteristics, multivariate analysis identified previous MI (HR 3.46; 95% CI 1.35–8.84; p=0.010) and PCI in the LAD (HR 2.81; 95% CI 1.21–6.51; p=0.016) as independent predictors of one-year MACE (Figure 1). Independent predictors of TLR at one year (Table 6) were previous CABG (HR 5.48; 95% CI 1.34–22.35; p=0.018) and PCI in the LAD (HR 4.76; 95% CI 1.11–20.36; p=0.035).
Patients who underwent PCI with DEB of the proximal LAD (n=49) had a higher cardiovascular risk profile, more often presenting with diabetes and/or hypertension (57% of diabetic and 92% of hypertensive patients undergoing PCI of the LAD vs. 38% of diabetic and 79% of hypertensive patients undergoing PCI of other vessels, p=0.024 and p=0.051, respectively). Patients with previous MI (n=71) more often had a history of coronary revascularization (90% with previous PCI and 32% CABG vs. 64% previous PCI and 11% CABG among those with no previous MI, p<0.001 and p=0.001, respectively), and naturally more often underwent the index PCI for in-stent restenosis (68% vs. 38% in those with no previous MI, p<0.001). Patients with a history of acute ischemic events more often presented left ventricular dysfunction (40% vs. 17% in those with no previous MI, p=0.006).
DiscussionThis study clearly demonstrated that DEB angioplasty in real-world patients with complex lesions is effective, with high rates of immediate angiographic success and low rates of MACE, including TLR at one-year follow-up. The results were equally good for in-stent restenosis and de novo CAD.
In recent years, DEBs have proved to be a valid alternative for in-stent restenosis following the good results achieved in clinical trials.5,6 Despite the fact that theoretically DEBs have the same potential disadvantages of acute recoil and late negative remodeling as conventional balloons, late luminal loss during follow-up has been consistently low in studies on DEBs (approximately 0.2 mm).9 PACCOCATH ISR I5 was the first randomized trial to compare DEBs with conventional balloons for in-stent restenosis and showed that DEB angioplasty was associated with a low incidence of restenosis and in-segment late luminal loss of only 0.03±0.48 mm at six months, significantly less than with conventional balloon angioplasty (p=0.002). PEPCAD II compared the safety and efficacy of treatment of BMS restenosis using DEBs vs. angioplasty with Taxus™ paclitaxel-coated stents. Here again, the primary endpoint, late luminal loss at six months, was lower in the group treated with DEBs (0.17±0.42 vs. 0.38±0.61 mm, p=0.03). One limitation of these studies is that the primary endpoint was late luminal loss, which is only a surrogate endpoint of angioplasty outcomes. In the case of PEPCAD II, use of this measure to compare two different strategies (balloon vs. stent) puts stent angioplasty at a disadvantage since calculation of late luminal loss depends directly on acute gain, which is always greater with stenting, and the greater the acute luminal gain, the greater the late luminal loss. It is thus to be expected that balloon angioplasty is associated with less luminal loss during follow-up.10 Nevertheless, both of these studies showed favorable clinical outcomes, with a lower rate of MACE for DEB angioplasty,5,6 a difference that was maintained at two-year11 (46% conventional balloon vs. 11% DEB, p=0.001) and five-year follow-up12 (59.3% conventional balloon vs. 27.8% DEB, p=0.009), mainly due to reduced need for TLR (38.9% conventional balloon vs. 9.3% DEB at five years, p=0.004).
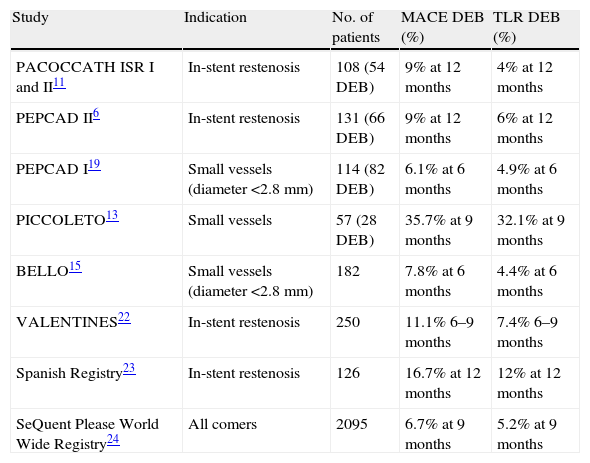
The present study analyzed MACE in a high-risk cohort with complex lesions. The combined incidence of events was 14% at 12 months, higher than in randomized trials (9% in PACCOCATH ISR I and II and in PEPCAD II).6,11 On the other hand, the rate of TLR was similar – 5% in our study compared to 4% in PACCOCATH ISR I and II and 6% in PEPCAD II.6,11 These results may reflect the fact that ours was an all-comers population that included patients with MI (30%), left ventricular dysfunction (21%) and chronic renal failure (9%), conditions that were generally criteria for exclusion in previous studies. The higher rates of death and MI during follow-up thus reflect the greater complexity of our patients. Table 7 summarizes clinical outcomes of DEB angioplasty in the main randomized trials and registries to date.
Main studies and registries on percutaneous coronary intervention with drug-eluting balloons.
| Study | Indication | No. of patients | MACE DEB (%) | TLR DEB (%) |
| PACOCCATH ISR I and II11 | In-stent restenosis | 108 (54 DEB) | 9% at 12 months | 4% at 12 months |
| PEPCAD II6 | In-stent restenosis | 131 (66 DEB) | 9% at 12 months | 6% at 12 months |
| PEPCAD I19 | Small vessels (diameter <2.8 mm) | 114 (82 DEB) | 6.1% at 6 months | 4.9% at 6 months |
| PICCOLETO13 | Small vessels | 57 (28 DEB) | 35.7% at 9 months | 32.1% at 9 months |
| BELLO15 | Small vessels (diameter <2.8 mm) | 182 | 7.8% at 6 months | 4.4% at 6 months |
| VALENTINES22 | In-stent restenosis | 250 | 11.1% 6–9 months | 7.4% 6–9 months |
| Spanish Registry23 | In-stent restenosis | 126 | 16.7% at 12 months | 12% at 12 months |
| SeQuent Please World Wide Registry24 | All comers | 2095 | 6.7% at 9 months | 5.2% at 9 months |
DEB: drug-eluting balloon; M: months; MACE: major adverse cardiac events; TLR: target lesion revascularization.
In our population, the results of DEB angioplasty were equally good in terms of efficacy for in-stent restenosis and de novo small-vessel disease. In the randomized PICCOLETO study,13 DEB angioplasty of small vessels was associated with higher rates of angiographic restenosis and MACE at nine months than Taxus™ stent angioplasty, which prompted early termination of the trial. The DEB used in that study was the first-generation DIOR™, which has different pharmacokinetics and bioavailability from the SeQuent® Please, with an inferior anti-restenotic effect.14 By contrast, the more recent randomized BELLO study15 on de novo lesions in small vessels (reference diameter <2.8 mm) showed DEB angioplasty to be superior to Taxus™ stenting in terms of the primary endpoint at six months (late luminal loss 0.08±0.38 mm vs. 0.29±0.44 mm, p=0.001), with low event rates of 7.8% MACE and 4.4% TLR in the DEB group. Percutaneous intervention in small vessels is associated with a high restenosis rate, and the use of DES to suppress neointimal hyperplasia is recommended whenever possible. The E-SIRIUS16 and C-SIRIUS17 trials showed that use of the Cypher™ stent for the treatment of long de novo coronary lesions in small vessels reduced restenosis at eight months compared to BMS. However, stents implanted in small arteries have a higher metal-to-artery ratio, which can increase the risk of restenosis.18 The fact that DEBs have no metal structure is thus a clear advantage. They also have the advantage of homogeneous release of the drug to the vessel wall, unlike DES, in which the drug is concentrated on the struts and thus cannot suppress neointimal hyperplasia between the struts or at the stent edges. Paclitaxel is the drug of choice for DEBs, as its strongly lipophilic properties mean that it remains on the vessel wall for up to a week, without the need for a controlled-release mechanism based on a metal platform and a polymer. This reduces the risk of chronic inflammation and thrombosis and the need for prolonged antiplatelet therapy.2,4
Among the limitations reported for DEB angioplasty are the fact that occasionally the target lesion cannot be crossed and that it can be associated with dissection or recoil, leading to a suboptimal angiographic result and need for bail-out stenting.2 In our study, the lesion could not be crossed in only three patients and the angiographic success rate was very high, with bail-out stenting required in only 3% of lesions, a much lower percentage than reported in previous studies (28% in PEPCAD I19 and 20% in BELLO15).
Assessment of predictors of MACE and TLR at one year demonstrated once again that prognosis was determined by patient characteristics and lesion complexity and that variables such as DEB length or diameter did not correlate with restenosis during follow-up. However, careful analysis of MACE-free survival curves shows that the curves for diabetes and DEB length (known predictors of TLR in previous studies20) diverged during follow-up (Figure 1), reflecting differences that could be significant with longer follow-up.
With regard to treatment of restenosis, the PEPCAD-DES study21 showed that the results of DEB angioplasty in treating DES restenosis were superior to plain balloon angioplasty, with lower rates of TLR (15.3% vs. 36.8%, p=0.005) and MACE (16.7% vs. 50.0%, p<0.001) at six months. In our registry, the event rate was no higher in interventions for DES restenosis, which confirms the efficacy of DEB angioplasty in these patients.
The study has certain limitations which are common in registries, of which the most important are the small sample size and short follow-up to date. It will be interesting to assess the long-term outcomes (beyond one year) of DEB angioplasty to confirm its safety and the persistence of the good results obtained.
ConclusionThis registry of two interventional cardiology centers shows that PCI with DEBs is effective in high-risk patients with complex lesions, with a high rate of angiographic success and a low rate of MACE, including TLR, at one-year follow-up. The results are equally good whether the intervention is for in-stent stenosis or native coronary disease.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Calé R, et al. Eventos cardiovasculares major após intervenção coronária percutânea com balão eluidor de fármaco: Resultados a um ano de um registo prospetivo multicêntrico. Rev Port Cardiol. 2013. http://dx.doi.org/10.1016/j.repc.2012.09.006.