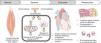
Short periods of myocardial ischemia followed by reperfusion induce a cardioprotective mechanism when the myocardium is subsequently subjected to a prolonged period of ischemia, a phenomenon known as ischemic preconditioning.
As well as its application in the myocardium, ischemic preconditioning can also be induced by brief interruptions of blood flow to other organs, particularly skeletal muscle. Transient ischemia induced noninvasively by inflating a cuff on a limb, followed by reperfusion, helps reduce the damage caused to the myocardium by interruption of the coronary circulation.
Remote ischemic preconditioning involves activation of humoral and/or neural pathways that open mitochondrial ATP-sensitive potassium channels in the myocardium and close mitochondrial permeability transition pores, making cardiomyocytes less vulnerable to ischemia-induced cell death.
This cardioprotective mechanism is now being translated into clinical practice, with positive results in several clinical trials in coronary artery bypass surgery, surgical repair of abdominal aortic aneurysms, valve replacement surgery and percutaneous coronary intervention. However, certain factors weaken the subcellular mechanisms of preconditioning – age, comorbidities, medication, anesthetic protocol – and appear to explain the heterogeneity of results in some studies.
Detailed understanding of the pathways involved in cardioprotection induced by ischemic preconditioning is expected to lead to the development of new drugs to reduce the consequences of prolonged ischemia.
Curtos períodos de isquemia do miocárdio seguida de reperfusão induzem um mecanismo de cardioproteção quando este é depois submetido a um período de isquemia prolongada, um fenómeno designado pré-condicionamento isquémico.
Além da sua aplicação local no miocárdio, o pré-condicionamento isquémico também pode ser induzido por breves interrupções da circulação sanguínea em outros órgãos, nomeadamente no músculo esquelético. De uma forma não invasiva, a indução de isquemia transitória através da insuflação de um braçal num dos membros, seguida de reperfusão, leva à diminuição dos danos causados no miocárdio pela interrupção da circulação coronária.
O pré-condicionamento isquémico remoto envolve a ativação de vias humorais e/ou neuronais que, atuando no miocárdio, provocam a abertura de canais de potássio mitocondriais sensíveis ao ATP e o encerramento do poro de transição de permeabilidade mitocondrial, tornando os cardiomiócitos menos sensíveis à morte celular causada pela isquemia.
Este mecanismo cardioprotetor pode já ser transposto para a prática clínica, havendo resultados positivos em vários estudos clínicos realizados na cirurgia coronária, cirurgia de reparação de aneurismas da aorta abdominal, cirurgia de substituição valvular e intervenção coronária percutânea. Contudo, existem alguns fatores que atenuando os mecanismos subcelulares do pré-condicionamento – idade, comorbilidades, medicação, protocolo anestésico – parecem explicar a heterogeneidade de resultados nalguns estudos.
Finalmente, é de esperar que a compreensão detalhada das vias envolvidas na cardioproteção induzida pelo pré-condicionamento isquémico possam permitir o desenvolvimento de novos fármacos que permitam reduzir as consequências da isquemia prolongada.
The human body is able to recruit various protective mechanisms in order to maintain homeostasis in response to a variety of aggressions.
When the coronary circulation is interrupted, the size of the resulting infarct is proportional to the duration of ischemia.1 Paradoxically, even early revascularization leads to tissue damage, a phenomenon known as ischemia-reperfusion injury,2 which is estimated to be responsible for up to 30% of infarct size.3 This has prompted a search for cytoprotective mechanisms that make the myocardium less vulnerable to such damage, not only in acute settings (as in revascularization in the context of acute coronary syndrome [ACS]) but also following surgical procedures that entail temporary interruption of the coronary circulation, particularly cardiac surgery with aortic clamping and heart transplantation.
In 1986, Murry et al.4 observed that in animals subjected to short episodes of coronary ischemia before prolonged occlusion of the same artery, infarct size was 25% of that seen in the control group. The authors proposed that short periods of non-lethal myocardial ischemia, followed by reperfusion, could protect the myocardium from subsequent prolonged ischemia, a phenomenon known as myocardial ischemic preconditioning.
Subsequent research into the mechanisms of ischemic preconditioning revealed two other phenomena: ischemic perconditioning5 and ischemic postconditioning,6 in which the cardioprotective stimulus is applied during and after prolonged coronary occlusion, respectively.
Przyklenk et al.7 extended the concept of ischemic preconditioning by showing that repeated brief occlusions of a coronary artery protect not only that artery's territory, as suggested by Murry, but also other parts of the myocardium. They called this intracardiac protection ‘regional ischemic preconditioning’. This opened up the possibility that such cytoprotective mechanisms could be induced by ischemia in remote organs, which was confirmed by reports of myocardial remote ischemic preconditioning (RIPC), initially induced by renal and mesenteric ischemia.8 Although this discovery was experimentally interesting, the kidney and, to a lesser extent, the intestine are vulnerable to damage from even brief periods of ischemia9 and are thus not suitable for clinical application in this context.
A major advance in myocardial RIPC came with the use of skeletal muscle as the ischemic stimulus.10 A tourniquet or inflatable cuff applied to a limb can induce RIPC without the need for invasive procedures or interruption of the blood supply to vital organs.10
Myocardial RIPC is thus a mechanism through which transient ischemia of distant vascular territories increases the resistance of cardiomyocytes to prolonged coronary ischemia and ischemia-reperfusion injury.
This article sets out to describe the pathophysiological mechanisms responsible for myocardial RIPC and to provide examples of possible clinical applications, examining the main clinical trials assessing its effectiveness. Inducing ischemia in a limb has greater clinical potential, since skeletal muscle is easily accessible and has high resistance to ischemia,12 and so this review of the literature will focus on induced by ischemia of skeletal muscle.
MethodologyWe searched PubMed for articles published between 1986 (when ischemic preconditioning was first described4) and December 2012 containing the terms “remote ischemic preconditioning” or “ischemic preconditioning at a distance”. Additional searches were performed in the Scopus and Cochrane databases. All articles considered relevant to the subject were included.
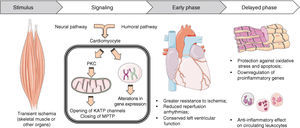
Pathophysiological mechanisms involved in remote ischemic preconditioningLike local preconditioning,13 the myocardial protection induced by RIPC occurs in two phases. The early phase or “first window” lasts around four hours, while the delayed phase (the “second window” of protection) begins >24 hours after the induction of ischemia and is sustained for at least 48 hours.14
In the early phase there are immediate alterations in the myocardium and coronary circulation, with increased diastolic flow15 and coronary vasodilation,16 which reduce infarct size and the risk of reperfusion arrhythmias11,17 (particularly extrasystoles and ventricular fibrillation and tachycardia18) and help preserve left ventricular function.19,20
The second window of cardioprotection depends on protein synthesis, which is consistent with the changes in gene expression seen in cardiomyocytes21 and leukocytes22 in the period following myocardial ischemia.
The pathophysiological mechanisms involved in RIPC are still not fully understood, but can be divided into three components: (i) the production or release of the effector(s) in the ischemic tissue; (ii) the mechanisms of communication between the distant territory and the myocardium; and (iii) the induction of a cardioprotective response (Figure 1).
Transient periods of ischemia-reperfusion trigger the production and/or release of various substances by the ischemic tissue (Table 1), but there is as yet no agreement as to their relative importance. Depending on the site of the stimulus (renal, mesenteric or skeletal muscle), different protective substances and mechanisms may be involved, which prevents extrapolation of data from one experimental protocol to others.23 For example, hexamethonium, a cholinergic antagonist, abolishes protection induced by mesenteric ischemia but not that induced by renal8 or skeletal muscle ischemia.24
Substances implicated in the development of myocardial remote ischemic preconditioning.
| Renal ischemia | Mesenteric ischemia | Skeletal muscle ischemia |
| Adenosine76–79 | Bradykinin80,81 | Opioids30,87–89 |
| Cannabinoids18 | NO24,31 | |
| CGRP82–84 | Noradrenaline11 | |
| Opioids85,86 | ROS31,40,88 |
CGRP: calcitonin gene-related peptide; NO: nitric oxide; ROS: reactive oxygen species.
The cardioprotection afforded by these mediators occurs through neural and/or humoral mechanisms, and there is evidence for both.
The neural hypothesis postulates that substances produced in the remote ischemic territory act locally via afferent neural pathways, activating various efferent pathways that induce cardioprotection. In favor of this hypothesis is the fact that a limb used for preconditioning must be enervated, since cutting the femoral nerve abolishes or weakens the protection conferred by transient ischemia of a lower limb.25,26 Nicotinic receptor antagonists and reserpine, which inhibits uptake of neurotransmitters by synaptic vesicles, also weaken RIPC.11,14 Neurons of the dorsal motor nucleus of the vagus nerve appear to play a crucial role in cardioprotective RIPC, and activating these neurons even in the absence of muscle ischemia is sufficient to reproduce the effect of remote preconditioning.27
The humoral hypothesis, on the other hand, posits that the ischemic stimulus leads to the production of substances that enter the circulation and reach the myocardium, where they have a protective effect. Support for this view comes from the fact that the remote organ must be reperfused before the onset of coronary ischemia for the protective effect to be produced.8,28 This suggests that substances must be ‘washed out’ and reach the heart via the circulation before the ischemic event occurs.
Studies of heart transplantation in animal models also support the humoral hypothesis. In pigs undergoing RIPC before transplantation, infarct area following myocardial infarction (MI) was reduced in the donor heart.29 Since in this case the heart has no extrinsic innervation, it is likely that a humoral factor in the circulation is acting on the transplanted heart. Furthermore, in isolated rabbit hearts perfused with plasma from donor animals subjected to RIPC, a cardioprotective effect is seen with significantly reduced infarct size,30 which supports the idea that the plasma contains a cytoprotective substance.
Whatever mechanism is responsible, a kind of memory is involved, since the explanted heart retains the effect of RIPC to which it was subjected in vivo.19
Subcellular mechanismsAlthough the substances that induce cardioprotection have not been identified, more is understood of the effects on cardiomyocytes at the subcellular level. The initial step appears to involve the activation of myocardial protein kinase C (PKC)30 (Figure 1).
Mitochondrial ATP-sensitive potassium (KATP) channels open during transient ischemia of skeletal muscle. These are downstream of PKC in RIPC32 and depend on it for their activation.33 It is thus likely that protein G agonists trigger a cardioprotective signaling cascade that activates PKC and opens mitochondrial KAPT channels.
In pathophysiological terms, as ATP is depleted during ischemia, ion channels lose function, leading to intracellular Ca2+ accumulation, which further reduces ATP. Mitochondrial Ca2+ overload mainly occurs when ischemia is followed by reperfusion; although reintroduction of oxygen enables ATP production to resume, ischemia-reperfusion injury alters the mitochondrial electron transport chain, resulting in the production of reactive oxygen species (ROS). Increases in ROS and mitochondrial Ca2+ and falls in the mitochondrial membrane potential following ischemia-reperfusion result in opening of the mitochondrial permeability transition pore (MPTP),34 a polyprotein mitochondrial transmembrane channel that is absent or closed in physiological conditions. Opening of the MPTP in response to ischemia leads to the release of mitochondrial proteins, including cytochrome C, into the cytoplasm, which activate the caspase cascade. This, in conjunction with low ATP levels and changes in ion homeostasis, results in rupture of the plasma membrane and cell death.35
These mechanisms are counteracted by the opening of mitochondrial KAPT channels via PKC activation, which depolarizes the mitochondrial membrane,36 thereby reducing Ca2+ uptake and concentrations during reperfusion and maintaining mitochondrial integrity, and thus has a cardioprotective effect. It also reduces the activity of voltage-dependent ion channels and preserves ATP by reducing hydrolysis.37,38 ROS production increases during preconditioning,31 which as well as possibly reducing their concentrations during subsequent ischemia,39 increases the production of antioxidant enzymes that preserve mitochondrial function and reduce apoptosis.40
The importance of PKC is not limited to its effect on mitochondrial KAPT channels; it also acts on the MPTP. During preconditioning, PKC forms a complex with the MPTP, preventing the latter from opening and thus inhibiting cardiomyocyte apoptosis during ischemia-reperfusion.41
Although the above subcellular sequence is the dominant theory, it is only one of several that seek to explain the mechanisms of preconditioning. The large number of substances involved in RIPC make it difficult to produce a single theory, since they may have synergistic effects, or there may be redundancy in the subcellular pathways involved in cardioprotection, which makes it difficult to determine their relative importance.42
The delayed phase of ischemic preconditioningThe second window of protection is apparently triggered by changes in the expression of genes involved in the myocardium's response to oxidative and inflammatory injury (Figure 1).
Inflammatory reactions are heightened during reperfusion, with polymorphonuclear leukocytes accumulating in the myocardium and contributing to cardiac damage by release of ROS, proteases and leukotrienes.43 In humans, RIPC leads to anti-inflammatory changes in circulating leukocytes, suppressing genes encoding proteins involved in chemotaxis, adhesion and migration, exocytosis, apoptosis and innate immunity within 15 minutes of the RIPC stimulus and more so after 24 hours (second window RIPC).22
Besides its role in modulating mitochondrial function, PKC is also involved in regulating gene expression,44 and may be responsible for the changes that occur in the delayed phase of preconditioning. Unlike in cardiomyocytes 15 minutes after RIPC, after 24 hours genes involved in cytoprotection (Hsp73) and protection against oxidative stress (including Hadhsc, Prdx4, and Fabp4) are upregulated, whereas many proinflammatory genes (e.g. Egr-1 and Dusp 1 and 6) are suppressed.21
Li et al.20 showed that nuclear factor kappa-B (NF-κB), a redox-sensitive transcription factor that regulates various inflammatory genes including those coding for inducible nitric oxide (NO) synthase (iNOS) and inducible cyclooxygenase, is involved in RIPC. Although NF-κB during ischemia-reperfusion is detrimental through production of leukocyte adhesion molecules, cytokines and chemokines and increased infarct size, when activated following RIPC it has an adaptive effect on the heart within 24 hours. This is because there is a parallel increase in its own inhibitor, IκB, which reduces NF-κB activation following reperfusion, reducing infarct size and protecting left ventricular function. Following preconditioning a gradual increase in iNOS mRNA is also seen, reaching a peak at 24 hours. Knockout mice for the NF-κB and iNOS genes do not exhibit adaptation to ischemia.45
It thus appears that preconditioning reduces the inflammatory response during reperfusion by inducing NF-κB, which increases production of its own inhibitor, leading to iNOS transcription, which in turn increases NO production. The latter's role in the delayed phase is not known, but it probably has antiapoptotic and anti-inflammatory effects.46,47
Clinical applications of remote ischemic preconditioningCardioprotection through RIPC is a highly promising therapy and there are currently over a hundred clinical trials registered on the clinicaltrials.gov website.
If experimental results can be reproduced in clinical practice, RIPC could be induced by, for example, cycles of inflation and deflation of a cuff on a limb. This would be a simple, rapid, extremely inexpensive, noninvasive and nonpharmacological method that could be applied before percutaneous or surgical interventions in which coronary blood flow is to be interrupted.
Remote ischemic preconditioning as adjuvant therapy in cardiac surgery or percutaneous coronary interventionThe first clinical trial using RIPC was in children undergoing surgical correction of congenital heart defects,48 in which four cycles of 5-min lower limb ischemia using a blood-pressure cuff followed by 5-min reperfusion reduced postoperative troponin I levels, inotropic requirement and airway resistance.
A subsequent randomized trial using a similar RIPC protocol in 57 individuals undergoing elective coronary artery bypass grafting (CABG) produced similar results, with a reduction in troponin T levels in the first 72 hours after surgery.49
Since then, there have been several clinical trials of RIPC in CABG surgery (with and without extracorporeal circulation), surgical repair of abdominal aortic aneurysms, valve replacement surgery and percutaneous coronary intervention (PCI). The main trials are summarized in Table 2. The primary endpoint in most cases was release of troponins after surgery, which is associated with worse short- and long-term prognosis50,51 and is related to infarct area.52,53
Clinical trials on remote ischemic preconditioning.
| Trial | Surgical procedure | Ischemic stimulus | Anesthetic agents | Results |
| Cheung et al. (2006)48 | Repair of congenital cardiac defects in children under ECC | Leg, 4 cycles (5I+5R) | Induced with sevoflurane, maintained with fentanyl and isoflurane | Lower cTnI, lower inotropic requirement at 3 and 6 hours postoperatively and lower airway resistance at 6 hours |
| Ali et al. (2007)90 | Open abdominal aortic aneurysm repair | 2 cycles (10I+10R), 1st cycle in the right CIA and 2nd in the left CIA | Induced with propofol and remifentanil, maintained with desflurane | Lower cTnI and serum creatinine; lower incidence of MI and shorter ICU stay |
| Hausenloy et al. (2007)49 | Elective coronary surgery under ECC | Right arm, 3 cycles (5I+5R) | Induced with midazolam, propofol and etomidate or fentanyl, maintained with propofol | Lower troponin T at 6, 12, 24 and 48 hours after surgery; 43% reduction of the AUC |
| Hoole et al. (2009)91 | Elective PCI in adults with coronary disease | Arm, 3 cycles (5I+5R) | Not applicable | Lower cTnI and improvement in ST-segment alterations; lower incidence of postoperative chest discomfort and cardiac/cerebral events at 6 months |
| Hong et al. (2010)55 | Elective coronary surgery without ECC | Arm, 4 cycles (5I+5R) | Induced with midazolam e sufentanil, maintained with sevoflurane and remifentanyl | Reduction (not statistically significant) in cTnI |
| Li et al. (2010)92 | Valve replacement for rheumatic valve disease | Right leg, 3 cycles (4I+4R) | Induced with midazolam, maintained with fentanyl and isoflurane | Lower cTnI and lower incidence of ventricular fibrillation after surgery |
| Thielmann et al. (2010)93 | Elective coronary surgery under ECC in adults with 3-vessel coronary disease | Left arm, 3 cycles (5I+5R) | Induced with sufentanil and etomidate, maintained with isoflurane or propofol | Lower cTnI (peak, total and AUC) after surgery |
| Rahman et al. (2010)94 | Elective or urgent coronary surgery under ECC in adults with multivessel coronary disease | Arm, 3 cycles (5I+5R) | Induced with etomidate and fentanyl, maintained with propofol and alfentanyl, supplemented with enflurane or sevoflurane during ECC | No differences between the groups |
| Kottenberg et al. (2011)63 | Elective coronary surgery under ECC in adults with 3-vessel coronary disease | Left arm, 3 cycles (5I+5R) | Induced with sufentanyl and etomidate, maintained with isoflorane or propofol | Lower cTnI (peak, total and AUC) with isoflurane but not with propofol |
| Karuppasamy et al. (2011)95 | Elective coronary surgery under ECC | Left arm, 3 cycles (5I+5R) | Induced with remifentanyl and propofol, maintained with isoflurane before ECC and propofol during and after ECC | No differences in cTnI, BNP, CK-MB, or central venous concentrations of cytokines or growth factors |
| Venugopal et al. (2011)96 | Elective coronary surgery under ECC | Right arm, 3 cycles (5I+5R) | Induced with midazolam and etomidate or propofol, maintained with propofol or volatile agents | Lower absolute troponin T at 72 hours after surgery |
| Ghaemian et al. (2012)97 | Elective PCI in adults with coronary disease | Leg, 2 cycles (5I+5R) | Not applicable | Reduced intra-procedural chest pain and ST-segment deviation; lower troponin T at 24 hours |
| Prasad et al. (2012)98 | Elective PCI in adults with coronary disease | Arm, 3 cycles (3I+3R) | Not applicable | No differences in cTnT, hs-CPR or EPCs |
| Lomivorotov et al. (2012)99 | Elective coronary surgery under ECC | Arm, 3 cycles (5I+5R) | Induced with fentanyl and propofol, maintained with isoflurane and fentanyl | Reduced mean arterial pressure and vascular resistance; increased stroke volume; no differences in cardiac necrosis markers |
| Young et al. (2012)100 | Elective coronary surgery under ECC in high-risk patients | Arm, 3 cycles (5I+5R) | Induced with midazolam and fentanyl, maintained with propofol and isoflurane | No differences in cTnT, markers of acute renal injury or postoperative support requirements |
AUC: area under the curve; BNP: brain natriuretic peptide; CIA; common iliac artery; CK-MB: creatine kinase MB; cTnI: cardiac troponin I; ECC: extracorporeal circulation; EPCs: endothelial progenitor cells; hs-CPR: high-sensitivity C-reactive protein; I: ischemia (duration in min); ICU: intensive care unit; MI: myocardial infarction; PCI: percutaneous coronary intervention; R: reperfusion (duration in min).
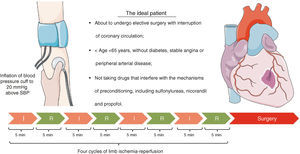
However, the results of trials on RIPC are not consistent, which may be due to differences in study protocols (such as the site for the preconditioning stimulus and number and duration of ischemia-reperfusion cycles), age, comorbidities, medication and anesthetic protocol during surgery.
Differences in preconditioning protocols and in study populations make it difficult to compare trials, which hampers attempts to establish a protocol that will afford maximum cardioprotection. In one study, on patients with stable angina and single-vessel disease undergoing elective PCI, RIPC induced by ischemia-reperfusion of both upper limbs actually led to increases in CK-MB and troponin I levels, particularly in those not taking statins.54 This may have been due to an increased inflammatory state following ischemia of skeletal muscle, which, in the absence of statins, worsened myocardial ischemia-reperfusion injury, rather than protecting against it. Furthermore, application of ischemia to both upper limbs simultaneously may be an excessive stimulus that does not confer benefit; most other trials have used ischemia of only one limb. It is also likely that the strength of the stimulus would differ between upper and lower limbs, due to their differing muscle mass.55
The patient's age may impose limitations to RIPC. Ageing leads to changes in cardiomyocytes, including reduced contractile function and weakened cardioprotective mechanisms,53 and the heart loses its sensitivity to preconditioning,56,57 which may limit its application in patients aged over 65.58
Comorbidities can also influence the effectiveness of preconditioning. For example, in a patient with stable angina, transient ischemia triggered by exertion have a natural preconditioning effect on the heart; several studies have shown that patients with angina in the 48 hours before MI have a better prognosis.58,59 Thus, in theory, patients with stable angina may not gain additional benefit from RIPC; nor would those with peripheral arterial disease, which simulates remote preconditioning.
Type 2 diabetes induces a state of chronic resistance to ischemia-reperfusion injury due to increases in levels of glycosylated proteins which, among other effects, alter mitochondrial function. One consequence is suppression of the MPTP, which, as mentioned above, is also an effect of RIPC. However, the additional cardioprotection induced by preconditioning is weakened in diabetic patients, since the same subcellular mechanisms are involved, and so RIPC does not appear to provide additional protection in these patients.59
The effect of RIPC is also influenced by patients’ medication, such as sulfonylureas, oral hypoglycemic agents that inhibit mitochondrial KATP channels. These drugs are associated with higher mortality following MI,60 which may be due to the fact that they prevent RIPC, which involves opening these channels.61 Chronic exposure to these agents thus makes the myocardium insensitive to RIPC.61 New sulfonylureas such as gliclazide, which are more specific to pancreatic beta cells, do not appear to increase cardiovascular mortality.62
Another factor influencing RIPC is the anesthetic protocol used during surgery. Kottenberg et al.63 compared different anesthetic regimens in patients undergoing RIPC during CABG with extracorporeal circulation. They found no differences when the anesthetic used was propofol, but lower troponin I levels were seen with isoflurane, which is consistent with data suggesting that volatile anesthetic agents have intrinsic preconditioning effects.64 It is thus possible that certain anesthetics provide cardioprotection when combined with skeletal muscle preconditioning through an additive or synergistic effect, but that other agents, such as propofol, do not.
A recent meta-analysis on RIPC in cardiac surgery confirmed a reduction in troponin levels after surgery, although there was considerable heterogeneity in the results, possibly due to the degree of blinding; studies with double blinding showed less marked reductions in cardiac necrosis markers postoperatively.65 Another meta-analysis, including nine studies with 704 patients, showed a statistically significant reduction in troponin release, even after excluding confounding factors such as the use of volatile anesthetic agents.66
Various aspects of RIPC need to be clarified in future studies, particularly the preconditioning protocol (duration, number of cycles and stimulus site). In addition, so far only the benefits of the early phase of RIPC have been tested, not the delayed phase.
On the basis of the different clinical trials analyzed, we propose a model for the application of RIPC in individuals about to undergo cardiac surgery (Figure 2).
A randomized double-blinded multicenter clinical trial, RIPHeart,67 designed to determine the benefits of RIPC is currently under way. It aims to recruit over 2000 patients undergoing cardiac surgery and its primary endpoint is a composite of all-cause mortality, non-fatal myocardial infarction, any new stroke, and/or acute renal failure.
Pharmacological ischemic preconditioningKnowledge of the pathophysiological mechanisms involved in RIPC may lead to the development of drugs that reproduce its effects, and hence new therapeutic strategies for preserving cardiac tissue subjected to ischemia. One drug currently under investigation is diazoxide, an activator of mitochondrial KAPT channels, administration of which before an episode of ischemia has been shown in experimental models to delay cardiomyocyte death and thus reduce infarct size.68 Only one clinical trial of use of this drug for preconditioning has been published to date; this showed that administration of diazoxide in cardioplegic solution during cardiac surgery is safe and improves mitochondrial function.69
However, some drugs already on the market owe some of their effects to activation of subcellular ischemic preconditioning mechanisms. One example is nicorandil, used in clinical practice as an antianginal agent, which has a dual action: as an NO donor it induces vasodilation of the epicardial coronary arteries, as well as opening mitochondrial KAPT channels, dilating coronary resistance vessels. In the IONA trial, treating stable angina with nicorandil reduced the combined endpoint of cardiovascular mortality, MI and hospitalization,70 which does not appear to be explained by its vasodilator effect alone. It is likely that by opening mitochondrial KAPT channels, nicorandil has a preconditioning effect on the myocardium, reducing ischemia-reperfusion injury.71
Ischemic preconditioning in organ transplantationAn area in which RIPC may be particularly valuable is heart transplantation. Before being transplanted, the organ is subjected to varying periods of ischemia, and ischemia-perfusion injury also occurs in the recipient.72 Preclinical trials show that if RIPC is induced in the recipient before transplantation, the cardioprotective effect is transferred to the donor heart.29
Although known pathophysiological mechanisms suggest that RIPC should be feasible before transplantation, no clinical trials have examined the possibility.
Clinical applications of other forms of remote ischemic conditioningTwo new forms of ischemic conditioning have been described in the last decade: perconditioning and postconditioning.
Remote ischemic postconditioning consists of cycles of limb ischemia-perfusion after myocardial reperfusion, such as immediately following primary PCI in patients with ST-elevation ACS. Pre-clinical trials have shown a cytoprotective effect similar to that of RIPC.6
Remote ischemic perconditioning involves the administration of the stimulus during myocardial ischemia, before reperfusion.5 It is an attractive clinical option since ischemic events cannot be predicted and perconditioning can be applied in acute situations such as MI. A clinical trial published in 2010 in the Lancet73 assessed 330 patients with ST-elevation ACS about to undergo PCI randomized during transport to hospital to standard therapy or remote conditioning by arm ischemia through four cycles of 5-min inflation and 5-min deflation of a blood-pressure cuff. Thirty days after PCI, the volume of viable myocardium compared to the area at risk was greater in the group who had undergone remote ischemic perconditioning.
Finally, protection against ischemia-reperfusion injury by preconditioning is not limited to the myocardium. Recent years have seen an exponential growth in research into this phenomenon, and there are reports of liver preconditioning by skeletal muscle ischemia74 and lung preconditioning by intestinal ischemia.75
ConclusionMyocardial RIPC is part of a complex web of interactions between and within organs through which the organism generates cytoprotective stimuli that increase its resistance to ischemia.
It is hoped that in the near future the application of this technique in various clinical contexts, including prior to cardiac surgery, following reperfusion therapy, and for heart transplantation, will help reduce ischemia-reperfusion injury and infarct area.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis work was funded by the Portuguese Foundation for Science and Technology (Projects PEst-C/SAU/UI0051/2011 and EXCL/BIM-MEC/0055/2012) through the Cardiovascular Research and Development Unit, and by European Commission Grant FP7-Health-2010 (MEDIA-261409).
Conflicts of interestThis work was funded by the Portuguese Foundation for Science and Technology (Projects PEst-C/SAU/UI0051/2011 and EXCL/BIM-MEC/0055/2012) through the Cardiovascular Research and Development Unit, and by European Commission Grant FP7-Health-2010 (MEDIA-261409).
Please cite this article as: Costa JF, Fontes-Carvalho R, Leite-Moreira AF. Pré-condicionamento isquémico remoto do miocárdio: dos mecanismos fisiopatológicos à aplicação na prática clínica. Rev Port Cardiol. 2013. http://dx.doi.org/10.1016/j.repc.2013.02.012