Imbalance between pro- and anti-inflammatory cytokines secreted from visceral adipose tissue (VAT) contributes to the pathogenesis of certain cardiovascular and metabolic disorders, including insulin resistance. Epicardial adipose tissue (EAT) is a form of VAT mainly concentrated along the coronary arteries. It has been shown in various studies that EAT thickness is positively correlated with cardiovascular disease. Due to its high worldwide prevalence, prevention and management of type 2 diabetes (T2D) has become a major public health challenge. Metformin, the most widely prescribed drug to treat T2D, has favorable effects on VAT and body weight. As metformin decreases VAT mass, in this prospective study we analyzed the possible positive effect of metformin on EAT mass, which is organ-specific VAT, and body mass index (BMI).
MethodsSubjects were selected from patients admitted to the internal medicine outpatient clinic. Newly diagnosed T2D patients treated with metformin monotherapy were analyzed and 40 patients were included. EAT thickness in the included patients was measured echocardiographically. BMI and EAT thickness were analyzed at the beginning (BMI0 and EAT0) and after three months of metformin monotherapy (BMI3 and EAT3).
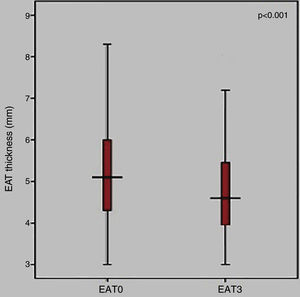
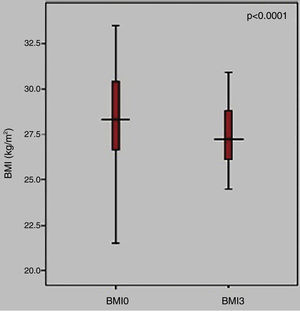
ResultsThere was a statistically significant decrease in EAT thickness after three months of metformin monotherapy (EAT0=5.07±1.33 mm vs. EAT3=4.76±1.32 mm; p<0.001). Furthermore, BMI was also significantly decreased (BMI0=28.27±2.71 vs. BMI3=27.29±2.10; p<0.0001).
ConclusionsIn this study we show that metformin monotherapy significantly decreases EAT thickness and BMI in T2D patients. This suggests that metformin could reduce the frequency of coronary atherosclerosis.
O desequilíbrio entre as citocinas pro e anti-inflamatórias segregadas pelo tecido adiposo visceral (TAV) contribui para a patogénese de certas doenças cardiovasculares e metabólicas, incluindo a resistência à insulina. O tecido adiposo epicárdico (EAT) é o TAV, concentrado principalmente ao longo das artérias coronárias. É previamente demonstrado em vários estudos que a espessura do EAT está positivamente correlacionada com a frequência de doença cardiovascular. Devido à alta prevalência mundial, a prevenção e tratamento da diabetes mellitus tipo 2 (DM2) tornou-se um grande desafio para a saúde pública. A metformina, o medicamento mais amplamente prescrito para o tratamento da DM2, tem efeitos favoráveis no TAV e no peso corporal. Como a metformina diminui a massa do TAV, neste estudo prospetivo, analisamos o possível efeito positivo da metformina na massa do EAT, que é o TAV do órgão e o índice de massa corporal.
MétodosOs indivíduos foram selecionados dos doentes admitidos no ambulatório de medicina interna. Doentes recém-diagnosticados com DM2 tratados com metformina em monoterapia foram investigados. Foram incluídos 40 doentes. A espessura do EAT dos doentes incluídos foi medida por ecocardiografia. A espessura do IMC e do EAT no início e três meses após a monoterapia com metformina foi analisada.
ResultadosHouve redução estatisticamente significativa na espessura do EAT após três meses de monoterapêutica com metformina (EAT0=5,07±1,33 mm versus EAT3=4,76±1,32 mm; p<0,001). Além disso, o IMC também diminuiu significativamente (IMC0=28,27±2,71 versus IMC3=27,29±2,10; p<0,0001).
Conclusões Neste estudo, verificámos que a metformina em monoterapia diminui significativamente a espessura do EAT e o IMC nos doentes com DM2, pelo que se pode concluir que a metformina pode reduzir a frequência de aterosclerose coronária.
Visceral adipose tissue (VAT), anatomically located around certain organs, is now accepted as an endocrine organ which secretes various adipokines that influence both nearby and distant tissues. Imbalance between pro- and anti-inflammatory adipokines secreted from VAT contributes to the pathogenesis of certain cardiovascular and metabolic disorders, including insulin resistance.1 Epicardial adipose tissue (EAT), an organ-specific VAT, is mainly found in the atrioventricular and interventricular grooves and along the major branches of the coronary arteries, and to a lesser extent around the atria, over the free wall of the right ventricle and over the apex of the left ventricle.2 Evidence is accumulating that bioactive molecules secreted by EAT can directly affect intima-media thickness and regulate vasomotor function.3 Furthermore, there is emerging evidence that EAT has a direct association with coronary atherosclerosis.4 In view of the putative proatherogenic effect of EAT, several clinical trials have shown a positive correlation between EAT thickness and coronary atherosclerosis.5–7
Although its exact mechanism of action is unknown, metformin is regarded as the gold standard treatment for type 2 diabetes.8 Beside its glucose-lowering effect, metformin also has a favorable effect on body weight, primarily due to reduction of VAT.9 In this study, we investigated the effects of metformin on EAT thickness and body mass index (BMI) in type 2 diabetes patients.
MethodsStudy populationIn this prospective observational study, we analyzed consecutive patients referred to the internal medicine outpatient clinic between September 1, 2015 and May 31, 2016. Patients with BMI <20 or >35 kg/m2 or poor echocardiographic window were excluded. A total of 47 newly diagnosed type 2 diabetes patients prescribed metformin monotherapy were selected. Clinical and demographic features of the selected patients were recorded. All patients were informed about the study and written consent was obtained. The study was approved by the local ethics committee.
Study protocolBefore initiation of metformin monotherapy, the BMI of all recruited patients was calculated (BMI0) and all underwent transthoracic echocardiography to assess EAT thickness (EAT0). Metformin 1000 mg twice daily was prescribed to all participants. After three months of metformin monotherapy, BMI was recalculated (BMI3) and transthoracic echocardiographic studies were repeated to measure EAT thickness (EAT3), and the resulting data were statistically analyzed.
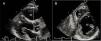
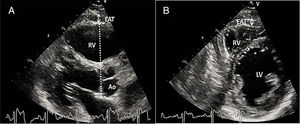
Measurement of epicardial adipose tissue thicknessAll included patients underwent detailed two-dimensional (2D), M-mode, Doppler, and tissue Doppler transthoracic echocardiography using standard techniques before beginning metformin monotherapy and after three months of treatment. The echocardiograms were performed by two experienced cardiologists who were blinded to the subjects’ clinical and demographic data. Each subject underwent 2D-guided M-mode transthoracic echocardiography using commercially available equipment (Aplio 500, Toshiba, Irvine, CA). Standard parasternal and apical views were obtained in left lateral decubitus position. EAT was visualized as echo-free space between the outer wall of the myocardium and the visceral layer of the pericardium (Figure 1). EAT thickness was measured perpendicularly on the free wall of the right ventricle during three cardiac cycles at end-diastole. Parasternal long- and short-axis views provided the most accurate measurement of EAT in the right ventricle, with optimal cursor beam orientation in each view. Maximum EAT thickness was measured at the point on the free wall of the right ventricle along the midline of the ultrasound beam perpendicular to the aortic annulus, used as an anatomic landmark for this view. For midventricular parasternal short-axis assessment, maximum EAT thickness was measured on the right ventricular free wall along the midline of the ultrasound beam, perpendicular to the ventricular septum at midchordal and tip of the papillary muscle level, as anatomic landmarks. The mean values from three cardiac cycles in each echocardiographic view were analyzed.
Statistical analysisThe statistical analysis was conducted using SPSS software, version 16.0 (IBM, Chicago, IL). Data were expressed as mean ± standard deviation for continuous variables and as counts and percentage for categorical variables. The Student's t test was used to compare continuous variables, while the chi-square and Fisher's exact tests were used to compare categorical variables. Correlations of continuous variables were assessed using Pearson correlation analysis. Values 0-0.3 indicated weak, 0.3-0.7 indicated intermediate, and 0.7-1.0 indicated strong correlation. A p value <0.05 was considered statistically significant.
ResultsSeven patients were excluded due to metformin intolerance during the follow-up period, and so a total of 40 patients were analyzed. Of the study population, 23 (57.5%) were male and mean age was 48.6±1.99 years. Fourteen patients (35%) had hypertension, 12 (30%) had hyperlipidemia and 13 (32.5%) were smokers. Mean fasting blood glucose was 280.38±14.88 mg/dl before beginning metformin monotherapy, falling to 129.01±17.00 mg/dl after three months of therapy.
EAT thickness was significantly decreased after three months of metformin monotherapy (EAT0= 5.07±1.33 mm vs. EAT3=4.76±1.32 mm; p<0.001) (Figure 2).
Furthermore, BMI was also significantly decreased after three months of metformin monotherapy (BMI0=28.27±2.71 vs. BMI3=27.29±2.10; p<0.0001) (Figure 3).
We also analyzed the correlation between the decrease in EAT (formulated as EAT0 - EAT3) and mean fasting blood glucose level before beginning metformin monotherapy and BMI0. A statistically non-significant positive correlation was found between decrease in EAT and mean fasting blood glucose before beginning therapy (r=0.19; p=0.056) and BMI0 (r=0.17; p=0.053).
DiscussionIn this study we demonstrated that metformin monotherapy significantly decreases EAT thickness and BMI as early as the third month of therapy.
Because of its vital endocrine function and close anatomical relation to the coronary arteries, many studies have set out to determine the effects of EAT on cardiovascular disease. Mahabadi et al. showed the existence of a relation between cardiovascular risk factors, myocardial infarction and EAT thickness,10 while Cavalcante et al. found a significant association between subclinical coronary atherosclerosis, metabolic syndrome, diastolic dysfunction and EAT thickness.11 In a recently published study, Abazid et al. also concluded that higher EAT volume is associated with greater coronary artery calcification,12 and Wu et al. showed that EAT thickness is positively correlated with obstructive coronary artery disease (CAD).13
Although the exact mechanism is unknown, several hypotheses have been put forward to explain this relation. EAT is a metabolically active fat depot which is rich in proinflammatory and proatherogenic cytokines including interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α.14 In a recent study, Vrselja et al. demonstrated that TNF-α levels were increased in CAD patients.15 Furthermore, Eiras et al. postulated that the extent of CAD was associated with the expression of IL-6 mRNA in EAT.16
EAT shares the same embryologic origin as mesenteric and omental fat.17,18 They all encase viscera in close contact, with no fascial barrier.19 Hence it has been suggested that EAT may have endocrine and paracrine effects on the adjacent coronary arterial wall that could result in atherosclerotic plaque development.20–22 These findings highlight the potential role of increased EAT thickness in atherosclerosis. It could therefore be postulated that decreased EAT would produce less proinflammatory and proatherogenic cytokines and be less likely to cause atherosclerosis.
Metformin is now accepted as the first-line drug for use as monotherapy in type 2 diabetes patients. It is thought to affect certain aspects of the body's metabolism. It inhibits hepatic gluconeogenesis, augments peripheral glucose uptake, and reduces insulin demand.8 Various studies have also investigated the effect of metformin on body weight and visceral adipose tissue. In a recent study, Alfaras et al. showed in mice that metformin significantly reduces body weight within the first weeks of treatment, without affecting food consumption or energy expenditure.23 Yanovski et al. showed that metformin significantly reduced body weight in children with insulin resistance,24 while Tokubuchi et al. demonstrated that metformin treatment caused significant reductions in visceral fat mass, probably through a potential shift of fuel resource into fat oxidation and upregulation of thermogenesis.25
Although there have been many studies on the effects of metformin on body weight and VAT, there is hardly any data about its effect on EAT. In our study, we showed that metformin significantly reduces BMI and EAT thickness. Magnetic resonance imaging (MRI) and computed tomography (CT) are the gold-standard techniques for quantifying EAT volume. Due to the difficulty in standardizing localization of the measurement site, reference values for EAT assessed by CT are uncertain.26 Iacobellis et al. were the first to propose that transthoracic echocardiography could be an easy and reliable imaging method for EAT prediction.27 MRI and transthoracic echocardiography provide comparable results for detection of EAT volume.28 Hence measurement of EAT by transthoracic echocardiography is practical, cost-effective, safe, rapid, and well correlated to gold-standard techniques.29
Study limitationsAs this is the first study to investigate the effect of metformin on EAT thickness, it is subject to some limitations, the main ones being its relatively small sample size and the lack of data on inflammatory markers. Due to financial constraints we were unable to analyze the possible effects of inflammation on the results. Larger-scale and more detailed studies should be designed to clarify the exact mechanisms underlying the favorable effects of metformin on EAT thickness and BMI.
ConclusionTo the best of our knowledge, our study demonstrates for the first time in the literature that metformin significantly reduces EAT thickness in newly diagnosed type 2 diabetes. Because EAT volume is closely related to atherosclerosis, it could be concluded that EAT is a modifiable risk factor for atherosclerosis and that metformin could significantly reduce the frequency of coronary atherosclerosis. Metformin could even become the aspirin of the 21st century. Further studies on a larger scale are needed to support these findings.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was presented as an oral presentation at the 32nd Turkish National Cardiology Congress (October 20-23, 2016).