An 83-year-old man with severe organic mitral regurgitation underwent mitral valve surgery with implantation of a biologic prosthesis. Four months later he presented with hemolytic anemia and heart failure due to severe paravalvular regurgitation. Since the patient refused surgery, the paravalvular leak was closed percutaneously using two Amplatzer devices, with angiographic and clinical success. Two months after the intervention he developed heart failure again and embolization of one of the devices was documented, with significant worsening of paravalvular regurgitation. A redo percutaneous closure was attempted but although initially promising, was ultimately unsuccessful as heart failure symptoms and hemolytic anemia persisted. Surgical correction was the final solution for this case.
This is the second case of late device embolization reported in the literature and highlights the importance of careful long-term follow-up of such patients, as late complications, although rare, may occur.
Um homem de 83 anos com insuficiência mitral orgânica severa foi submetido a cirurgia de substituição valvular com implantação de uma prótese biológica. Quatro meses após a cirurgia desenvolveu anemia hemolítica e insuficiência cardíaca justificadas por uma regurgitação paravalvular mitral severa. Após ter sido recusada cirurgia, foi realizado encerramento percutâneo, usando dois dispositivos Amplatzer, com bom resultado angiográfico e clínico. Dois meses após a intervenção desenvolveu novamente insuficiência cardíaca. Documentou-se a embolização de um dos dispositivos com consequente agravamento da regurgitação paravalvular. Foi tentado novo encerramento percutâneo, mas apesar de alguma melhoria inicial, manteve insuficiência cardíaca e anemia hemolítica. Efetuou-se, então, correção cirúrgica da regurgitação paravalvular com resolução do quadro clínico. Este é o segundo caso de embolização tardia de dispositivo descrito na literatura e demonstra a necessidade de um seguimento cuidado destes doentes, porque, apesar de raras, as complicações tardias ocorrem.
An 83-year-old man was referred for heart failure (HF) symptoms (New York Heart Association class II). Transthoracic echocardiography (TTE) revealed severe organic mitral regurgitation, due to ruptured chordae tendineae (effective regurgitant orifice area of 0.55 cm2) with mild left ventricular enlargement and normal ejection fraction (67% by Simpson's method).
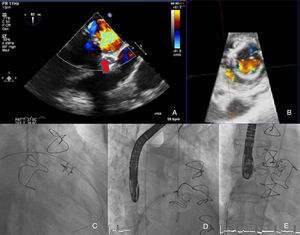
Surgical replacement of the mitral valve with a biologic prosthesis (31 mm Carpentier Edwards) was performed. Four months later, he was admitted for symptomatic hemolytic anemia (hemoglobin 9 g/dl, high lactate dehydrogenase and undetectable haptoglobin). Transesophageal echocardiography (TEE) showed severe antero-lateral paraprosthetic regurgitation (Figure 1A and B). Reoperation was proposed and refused by the patient. For this reason, and also because of his high surgical risk, it was decided to perform percutaneous paravalvular leak (PVL) closure.
The procedure was performed under general anesthesia, via the left femoral vein under fluoroscopic and TEE guidance. After transseptal puncture, a NuMED Tyshak II 12 mm×40 mm balloon was inflated at low pressure until complete closure was achieved and used to assess the dimension of the leak and choose the appropriate device. Two 7F sheaths (AGA Medical Corporation) were advanced across the mitral leak and an Amplatzer Vascular Plug III was deployed (Figure 1C) but still attached to the delivery cable. As the second sheath was occlusive (Figure 1D), it was decided to place a round device, an 8/6 mm Amplatzer Duct Occluder (Figure 1E). Only residual paraprosthetic regurgitant flow persisted and both devices were released. There was significant clinical improvement.
Two months later, the patient was again admitted for HF symptoms.
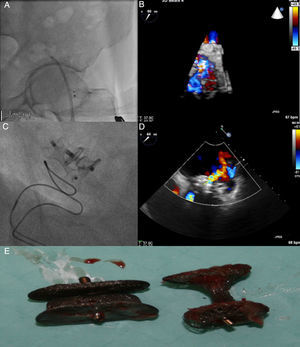
TEE revealed severe paraprosthetic regurgitation in an anterior position, close to the previously implanted devices (Figure 2B). Only one of the two devices was identified. Fluoroscopy confirmed embolization of the Amplatzer Duct Occluder to the left femoral artery (Figure 2A). Percutaneous removal was attempted but was unsuccessful and surgical removal was performed.
Fluoroscopy showing embolization of the Amplatzer Duct Occluder to the left femoral artery (A). Transesophageal echocardiography (TEE) revealed severe paraprosthetic regurgitation (B). An Amplatzer Muscular VSD Occluder was implanted near to the previous device (C). Three months later, TEE showed moderate paraprosthetic regurgitation (D). The devices were surgically removed (E).
Because the patient remained unwilling to undergo surgical correction, after a multidisciplinary discussion, a redo percutaneous closure was tried as a final option. The leak was crossed anteroseptally with a 280 cm, 0.035″ hydrophilic Terumo guidewire. As it was difficult to cross the leak with the 45° curve Amplatzer 7F sheath an arteriovenous loop was performed, snaring the wire in the aorta, through the femoral artery. An 8 mm Amplatzer Muscular VSD Occluder was implanted near to the previously implanted Amplatzer Vascular Plug III (Figure 1C). TEE at the end of the procedure showed only mild to moderate paraprosthetic regurgitation. There was prompt favorable clinical evolution.
Three months later, the patient again developed HF symptoms and hemolytic anemia. TTE (Figure 2D) showed the two devices in an anterior-lateral position and moderate paraprosthetic regurgitation. The mitral prosthetic valve was functioning normally.
At this point, cardiac surgery was presented to the patient as his only option and he eventually accepted. Device removal (Figure 2 E) and paravalvular leak suture were successfully performed.
Since then the patient has remained asymptomatic. TTE revealed the biologic mitral prosthesis functioning normally (mean gradient 6 mmHg), with no paravalvular regurgitation and with normal left ventricular function.
DiscussionPVL is a serious but rare complication1 that following mitral valve replacement has an incidence of 7–17%.2 Between 1% and 5% of mitral valve leaks are large and present serious consequences such as severe HF symptoms (90% of cases), hemolysis (33–75%) or endocarditis.1,2
Mitral PVL may be classified as early (first six months), mostly associated with technical aspects of the surgery, or late, due to suture dehiscence induced by infection or annular calcification.3
Leaks that are large or associated with symptoms are indicated for intervention.4,5 Reoperation, which has a mortality rate approaching 16%,1 is recommended when the leak is associated with endocarditis or severe symptoms like HF or hemolysis with need for blood transfusions.4 In the event of surgical contraindications or in high-risk patients, medical treatment is an option.4 Transcatheter mitral PVL closure is a valuable option6 and usually provides lasting symptomatic relief, depending on residual regurgitation, with low mortality (1.4–2%),2 although experience is limited.4,7,8 However, technical success is variable (60–90%) and the need for reintervention may be as high as 40%,1 but complications are rare.2 Device embolization occurs, mostly soon after the procedure,9 in 0.7–4% of cases.2
We present a case of severe organic mitral regurgitation unsuitable for repair, leading to valve replacement. Soon after surgery the patient developed symptomatic mitral PVL and percutaneous transcatheter closure was attempted.
Two months later, reintervention was needed due to late device embolization. In this patient the leak shape (oblong) and dimensions required the placement of two devices that required two sequentially deliveries. Unfortunately the devices currently in use were designed for closure of other cardiac defects, which may explain the late device embolization.
Redo transcatheter closure was attempted with a different device, but success was only partial. We decided to wait a few months for complete endothelization of the devices, believing that this might reduce regurgitant volume. However, the opposite happened, as the regurgitation worsened. Surgical repair was eventually the only solution.
With this case we aim to show the importance of percutaneous mitral PVL closure in some patients. Although it is not free of complications and repeat interventions and multiple devices may be necessary, this is usually a safe and effective procedure. Moreover, this case demonstrates the need for close follow-up of these patients because late embolization, although rare, may occur. In fact, to our knowledge, this is only the second case report in the literature.9
Conflicts of interestThe authors have no conflicts of interest to declare.