Transient apical dyskinesia syndromes present features similar to acute coronary syndromes, but with normal coronary arteries and rapid complete resolution of wall motion alterations. We report the case of a 73-year-old woman who was admitted to hospital because of typical chest pain at rest after her brother's death. She had had a pacemaker implanted in 2001. Troponin levels were elevated and apical hypokinesia was shown by ventriculography and echocardiography, with normal coronary arteries. Evolving ECG alterations were observed in spite of the continued pacing rhythm. All these alterations were fully resolved after discharge. This case shows that, even in the presence of a pacemaker, evolving ECG alterations can be observed in Takotsubo syndrome.
As síndromes de discinesia apicais transitórias apresentam características semelhantes às síndromes coronárias agudas, embora com artérias coronárias normais e resolução completa rápida das alterações da contractilidade segmentar. Apresentamos o caso de uma mulher de 73 anos que recorreu ao hospital em virtude de dor torácica na sequência da morte do irmão. Tinha pacemaker implantado desde 2001. Verificou-se elevação da troponina e foi detetada hipocinesia apical através de ecocardiograma, com coronárias normais. São reveladas alterações evolutivas no ECG, apesar do ritmo de pace continuado. Todas estas alterações foram totalmente resolvidas, após a alta hospitalar. Este caso mostra que, mesmo na presença de um pacemaker, as alterações evolutivas no ECG podem ser observadas na síndrome de Takosubo.
Transient apical dyskinesia, also known as apical ballooning, broken heart syndrome and Takotsubo cardiomyopathy, is a newly recognized entity. It was described in Japan by Sato et al.1,2 in 1990, and subsequently by other authors in other locations and races.3
This syndrome presents features similar to acute coronary syndromes. The main distinguishing characteristics are the presence of normal coronary arteries and the rapid and complete resolution of wall motion alterations. Prognosis is excellent compared with classic myocardial infarction.3,4 An episode of stress (emotional or physical) is often associated with the development of this process, usually in women.1–4 However, new findings have recently been published of alterations other than in the left ventricular apex, including midventricular,5,6 basal7 and biventricular alterations8 and previous coronary artery disease.9 Our aim is to report the electrocardiographic appearance of this entity when a pacemaker rhythm is present.
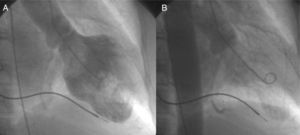
Case reportA 73-year-old woman was admitted to our coronary care unit complaining of typical chest pain at rest that developed two hours before, after she was informed of her brother's death. Six years before, a VVIR pacemaker was implanted due to sick sinus syndrome with occasional bursts of atrial fibrillation. On admission, myocardial necrosis markers were elevated, with first troponin I measurement of 0.46 IU/ml, with a peak of 0.75 IU/ml (normal <0.2 IU/ml) 11 hours after pain onset. Creatine kinase remained within normal limits. A transthoracic echocardiogram showed left ventricular ejection fraction (LVEF) of 37% with apical akinesia. A previous control echocardiogram, one month before, was available showing no wall motion abnormalities and normal LVEF (70%). Cardiac catheterization was performed three days later, showing normal coronary arteries and severe apical hypokinesia (Figure 1). In-hospital stay was seven days. Full recovery of left ventricular wall motion was observed on an echocardiogram performed 51 days after the onset of symptoms. During a follow-up of 24 months, the patient remained free of symptoms of ischemic cardiomyopathy and the echocardiogram was unchanged.
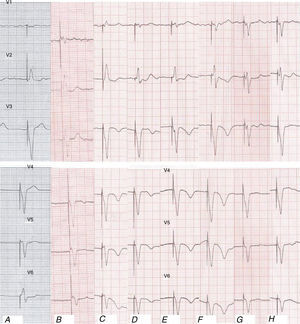
On admission four new negative T waves were observed which were absent in the previous electrocardiogram, in spite of the VVI pacing rhythm for left bundle branch block (LBBB). The deepest was in V4 (4 mm), with an anterolateral distribution. A total of six negative T waves were seen. The second tracing, six hours later, displayed seven negative T waves, with the same distribution. The third ECG, 48 hours later, when the patient was asymptomatic, shows nine more, deeper, negative T waves. The deepest was in V4 (9 mm). QT also widened, due to the size of the negative T waves. At discharge, the ECG was similar. During follow-up, two months later, normalization with respect to the previous electrocardiogram was seen. Her latest ECG shows a similar pattern, with pacemaker rhythm and only two negative T waves (Figure 2).
Electrocardiograms (ECG) (leads V1–V6) showing repolarization changes in spite of left bundle branch block: (A) previous ECG; (B) ambulance ECG, with chest pain; (C) admission ECG; (D) ECG six hours after admission; (E) 48 hours after admission; (F) ECG at discharge; (G) follow-up ECG two months later; (H) follow-up ECG 16 months later.
Transient myocardial dysfunction represents a special form of myocardial stunning.10 Its pathophysiology is not understood, although several theories have been proposed, including left ventricular outflow tract obstruction,11 anatomic coronary alterations,12 coronary microvascular dysfunction, transient intracoronary thrombus, vasospasm, myocarditis and catecholamine excess (related to mental stress).10 Various clinical criteria have been proposed in order to standardize diagnosis. The Mayo Clinic criteria require transient hypokinesis, akinesis or dyskinesis of left ventricular segments, with regional wall motion abnormalities that extend beyond a single epicardial vascular distribution; absence of obstructive coronary artery disease or angiographic evidence of acute plaque rupture; ECG abnormalities or elevated cardiac troponin; and absence of recent significant head trauma, pheochromocytoma, or myocarditis.13 Our patient's transient wall motion episode met the Mayo Clinic criteria, and in addition the pain began after emotional stress.
Serial electrocardiographic findings of transient apical dyskinesia have been studied and divided into four phases from onset until complete recovery, months later.14 Our aim with this case was to present a similar pattern in a patient with a pacemaker. It is known that the presence of LBBB can mask ECG findings. However, in coronary artery disease, ECG changes can still be assessed using other methods, such as the Sgarbossa criteria for ST-elevation myocardial infarction.15 Since ECG changes are included in the diagnostic criteria of this entity,13 the ability to recognize these changes in spite of the presence of previous LBBB may be useful.
ConclusionEven in the presence of previous LBBB it is possible to assess ECG alterations in the evolution of transient apical dyskinesia. This is important since ECG changes are included in the diagnostic criteria of this entity.
Ethical disclosuresRight to privacy and informed consentThe authors declare that no patient data appear in this article.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Conflicts of interestNone.