Brugada syndrome is an inherited cardiac condition with the potential for development of life-threatening arrhythmias in relatively young individuals without significant structural cardiac abnormalities. The condition is characterized by a distinct coved-type ST segment elevation in the right precordial leads (V1-V3). This hallmark pattern (type 1) is often dynamic and sometimes concealed, and may be unmasked in certain conditions or under the effect of certain agents, which include variation of sympathovagal balance, hormones, metabolic factors and drugs. These factors may not only modulate electrocardiographic morphology and induce the characteristic type 1 pattern, but also predispose to ventricular arrhythmias. The risk of malignant arrhythmias in acute events with induced type 1 pattern may be imminent, particularly if the patient in fact has Brugada syndrome. The physician should be aware of the modulating factors that may underlie a Brugada pattern, and be able to recognize, identify and promptly correct them. The mechanisms responsible for the type 1 pattern and possible associated ventricular arrhythmias induced by these modulating factors have attracted growing attention and interest. Furthermore, not all induced Brugada ECG patterns are observed in patients with Brugada syndrome, existing the possibility for acquired Brugada patterns/syndrome and Brugada phenocopies. This paper reviews the modulating factors associated with induced type 1 pattern as possible causes of arrhythmogenesis, particularly in Brugada syndrome patients, describes some of the probable underlying mechanisms, and discusses the concepts of acquired Brugada syndrome and Brugada phenocopies.
A síndrome de Brugada é um distúrbio cardíaco congénito com o potencial para o desenvolvimento de arritmias fatais em indivíduos relativamente jovens sem anomalias estruturais cardíacas grosseiras. Essa condição é caracterizada por uma distinta elevação do segmento-ST com concavidade superior (tipo 1) nas derivações precordiais (V1-V3). Esse peculiar padrão tipo 1 é frequentemente dinâmico e por vezes dissimulado, podendo ser desmascarado em certas condições ou sobre o efeito de alguns agentes que coletivamente envolvem e incluem o equilíbrio simpatovagal, hormonas, fatores metabólicos e agentes farmacológicos. Não só esses fatores podem modular a morfologia eletrocardiográfica e induzir o característico padrão tipo 1, como também predispor a arritmias ventriculares. Esse risco de arritmias malignas em eventos agudos pode ser iminente e possivelmente presente, particularmente se o paciente apresentar uma efetiva síndrome de Brugada. O clínico deverá estar ciente, reconhecer, identificar e prontamente corrigir esses possíveis fatores modeladores que possam estar subjacentes a um padrão de Brugada. Os mecanismos responsáveis por esses padrões induzidos do tipo 1 e possíveis arritmias ventriculares associadas por esses fatores moduladores têm igualmente trazido crescente atenção e interesse. Ademais, nem todos os padrões de Brugada induzidos ocorrem em pacientes com síndrome de Brugada, existindo a possibilidade para padrões/síndrome adquirida e fenocópias de Brugada. Este artigo faz uma revisão dos fatores moduladores associados ao padrão tipo 1 induzido, como possíveis fontes para arritmogénese, particularmente em pacientes com síndrome de Brugada, descreve alguns dos prováveis mecanismos subjacentes e aborda os conceitos de síndrome de Brugada adquirida e fenocópias.
Brugada syndrome (BrS) was first described as a distinct clinical entity in 1992 by Pedro and Josep Brugada1. Considered a primary electrical heart disease, BrS is an inherited cardiac condition electrocardiographically characterized by a distinct coved-type ST segment configuration (type 1) in the right precordial leads in the absence of significant structural heart disease, and typically presents a high risk of sudden cardiac death (SCD) secondary to polymorphic ventricular tachycardia (PVT) and/or ventricular fibrillation (VF)2,3. It is estimated to be responsible for at least 4% of all sudden deaths and at least 20% of sudden deaths in patients with structurally normal hearts3. However, this concept of the structurally normal heart in BrS has been challenged4,5.
The prevalence of BrS with a type 1 electrocardiogram (ECG) in adults is much higher in East Asian countries, where the syndrome is endemic, but in western countries the prevalence is lower5. It typically manifests during adulthood and is 8-10 times more prevalent in males than in females3,5. Most BrS patients are asymptomatic, representing a majority (around 63%) of newly diagnosed Brugada patients. When present, symptoms include ventricular tachycardia (VT)/VF or aborted SCD, syncope, nocturnal agonal respiration, palpitations, or chest discomfort. Unfortunately, sudden cardiac arrest (SCA) or SCD can be the first manifestation, frequently occurring without any preceding clinical sign5–8. These symptoms and arrhythmic events are more frequently observed at rest and during sleep, typically between 12 am and 6 am, and less frequently during the daytime9.
BrS displays autosomal dominant inheritance with incomplete penetrance. The SCN5A gene, which codes for the alpha subunit of the cardiac sodium channel Nav1.5, was the first gene found to be linked to BrS10. To date, more than 300 SCN5A gene variants related to BrS have been described, accounting for 18-28% of BrS cases. Soon, variants in other genes were found to be related to BrS, now making up a total of 18 genes. Mutations in these genes may result in a loss of function in cardiac sodium (INa) or calcium (ICa) channel currents, or in a gain of function in transient outward (Ito) or adenosine-triphosphate-sensitive (IK-ATP) potassium currents5.
The type 1 BrS ECG is often dynamic and sometimes concealed, and may be unmasked during febrile states, due to electrolyte imbalance, or under vagotonic conditions such as at rest or during sleep (but rarely during exercise), or under the effect of certain agents, such as sodium channel blockers (class IA and IC antiarrhythmic drugs). These modulating factors may not only induce a type 1 pattern but also predispose to associated malignant ventricular arrhythmias (VAs)3,5,11–15. Recently, many other drugs, including antidepressants, antipsychotics, anesthetics, antihistamines and cocaine, have also been implied in the induction of Brugada patterns, which represents a considerable challenge for physicians in clinical practice because of their potential for arrhythmic events16–19. Moreover, not all induced Brugada patterns occur in patients with BrS, existing the possibility for acquired Brugada patterns/syndrome and Brugada phenocopies (BrPs)5,7,16–20.
The purpose of this paper was to review the literature on the modulators (agents and conditions) associated with induced type 1 Brugada pattern, as possible causes of adverse events and arrhythmogenesis, particularly in BrS itself, and to describe some of the possible underlying mechanisms. It also presents some of the confounding factors that could account for an ECG abnormality similar to type 1 Brugada pattern, and discusses the concepts of acquired BrS and BrPs.
MethodsThe survey was conducted by searching the PubMed database for relevant Portuguese- and English-language studies published between January 1, 2012 and December 31, 2016, using the following search terms: ((“adverse effects”[Subheading] OR “acquired”[All Fields] OR “induced”[All Fields] OR “modulating”[All Fields] OR “iatrogenic disease”[MeSH] OR “drug-induced”[All Fields] OR “fever”[MeSH] OR “exercise”[MeSH] OR “water-electrolyte imbalance”[MeSH] OR “ethanol”[MeSH] OR “cocaine”[MeSH]) AND (“Brugada Syndrome”[MeSH] OR “brugada syndrome”[All Fields])) OR “brugada phenocopy”[All Fields], which yielded 359 articles.
An initial assessment of eligibility was made through titles and abstracts. Potentially relevant articles were retrieved and their full text was reviewed independently by the authors for final decision on inclusion. Unavailable and irrelevant articles were excluded. Additional relevant papers found in the reference lists of the articles retrieved from the initial selection were also included. A total of 93 articles made up the final study.
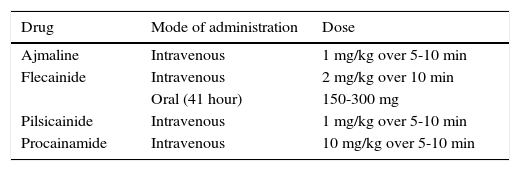
Brugada phenotype and diagnosisA pharmacologic challenge with one of the class I antiarrhythmic agents (Table 1), which block INa, may be used as a diagnostic tool for susceptible patients by unmasking the type 1 pattern5,11,16. The 2016 expert consensus on J-wave syndromes5 expresses concerns about the potential for overdiagnosis of BrS, particularly in patients displaying a type 1 pattern only after a drug challenge, contrasting with the previous 2013 consensus statement on inherited cardiac arrhythmias6. Thus, it is now suggested that development of a type 1 pattern with this test should be considered as probabilistic rather than binary in nature. The same may apply to the interpretation of a genetic test5.
Provocative agents used in drug challenge for diagnosis of Brugada syndrome.
| Drug | Mode of administration | Dose |
|---|---|---|
| Ajmaline | Intravenous | 1 mg/kg over 5-10 min |
| Flecainide | Intravenous | 2 mg/kg over 10 min |
| Oral (41 hour) | 150-300 mg | |
| Pilsicainide | Intravenous | 1 mg/kg over 5-10 min |
| Procainamide | Intravenous | 10 mg/kg over 5-10 min |
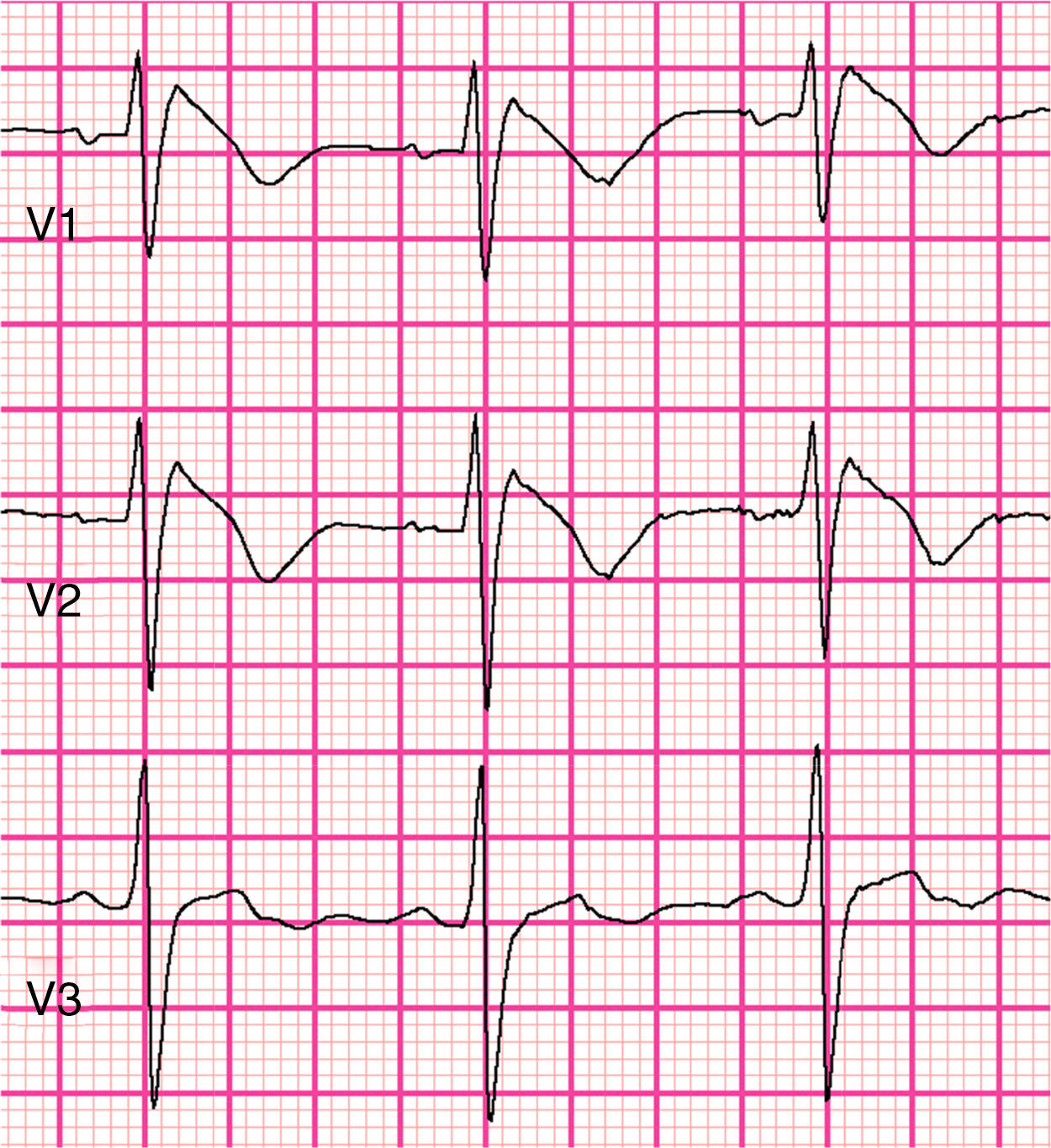
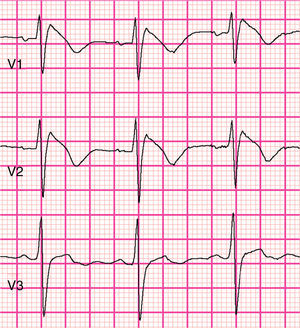
The 2016 consensus report5 recommends that only a type 1 (coved-type) ST-segment elevation is considered diagnostic of BrS (Figure 1), specifically an elevation of ≥2mm (0.2mV) in ≥1 right precordial leads (V1–V3) positioned in the 4th, 3rd, or 2nd intercostal space. Type 2 (saddleback-type) is characterized by ST-segment elevation of ≥0.5mm (generally ≥2mm in V2) in ≥1 right precordial lead (V1-V3), followed by a convex ST, and by a positive T wave in V2 and variable morphology in V1. Type 3 pattern is characterized by either a saddleback or coved appearance with an ST-segment elevation of <1mm. Type 2 or type 3 ST-segment elevation can be used for the diagnosis of BrS only if converted to type 1 with fever or pharmacologic challenge. However, when a type 1 ECG is unmasked using drug challenge, diagnosis of BrS should require that the patient also present with at least one of the following: documented VF or PVT, syncope of probable arrhythmic cause, a family history of SCD at <45 years old with negative autopsy, coved-type ECGs in family members, or nocturnal agonal respiration. A pharmacologic challenge may therefore be useful only when there is clinical suspicion of BrS in the absence of spontaneous type 1 ST-segment elevation. Alternatively, programmed ventricular stimulation inducing VT/VF with one or two premature beats may also support the diagnosis when the above clinical features are present5.
It is recommended that electrocardiographic recordings be obtained in the standard and superior positions for the V1 and V2 leads, because placement in more cranial positions (in the 3rd or 2nd intercostal space) increases the sensitivity of the ECG5. An alternative diagnostic tool, the full stomach test, in which ECGs are performed before and after a large meal, has been proposed for diagnosing BrS21. It is reasonable to assume that a spontaneous type 1 recorded by Holter at night or after a large meal has more diagnostic and prognostic value than a drug-induced type 15.
The criteria of the Proposed Shanghai BrS Score for the diagnosis of BrS are based on the ECG, clinical history, family history and genetic test results, but this score needs to be validated in further studies5.
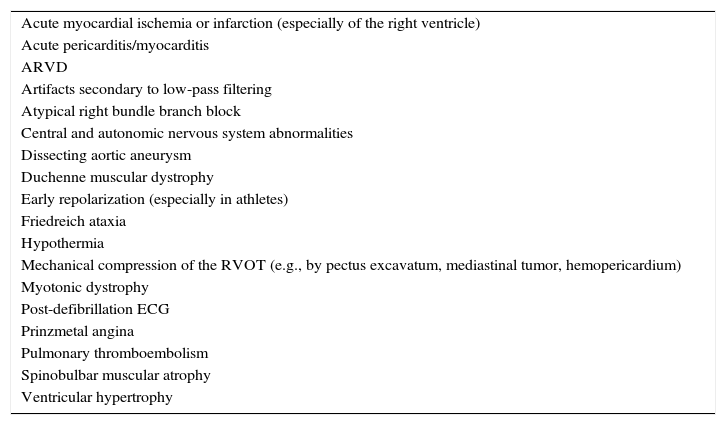
Differential diagnosis of Brugada syndromeOther circumstances can produce a type 1 Brugada-like ECG (ST-segment elevation mimicking a type 1 Brugada pattern) (Table 2)5,7,8. These are separate clinical entities with different pathophysiologies and prognosis and can thus be interpreted as confounding factors; they should be excluded before the establishment of a definitive diagnosis of BrS3,5,8.
Differential diagnosis of Brugada pattern.
| Acute myocardial ischemia or infarction (especially of the right ventricle) |
| Acute pericarditis/myocarditis |
| ARVD |
| Artifacts secondary to low-pass filtering |
| Atypical right bundle branch block |
| Central and autonomic nervous system abnormalities |
| Dissecting aortic aneurysm |
| Duchenne muscular dystrophy |
| Early repolarization (especially in athletes) |
| Friedreich ataxia |
| Hypothermia |
| Mechanical compression of the RVOT (e.g., by pectus excavatum, mediastinal tumor, hemopericardium) |
| Myotonic dystrophy |
| Post-defibrillation ECG |
| Prinzmetal angina |
| Pulmonary thromboembolism |
| Spinobulbar muscular atrophy |
| Ventricular hypertrophy |
Adapted from Antzelevitch et al.5 ARVD: arrhythmogenic right ventricular dysplasia; ECG: electrocardiogram; RVOT: right ventricular outflow tract.
The pathophysiology of BrS is only partially understood8. There are two main theories, the repolarization hypothesis and the depolarization hypothesis5,22,23.
The repolarization theory suggests that a decrease in inward currents (INa and ICa) and/or increase in outward currents (Ito) results in an outward shift in the balance of the ionic currents active at the end of phase 1 of the action potential (AP) in the right ventricular (RV) epicardium, where Ito is prominent. This results in loss of the AP dome and accentuation of the AP notch in the epicardium but not the endocardium, creating a transmural voltage gradient manifested by the characteristic Brugada ST-segment elevation on the ECG and dispersion of repolarization within the epicardium and transmurally. These repolarization abnormalities can precipitate the development of phase 2 reentry (local re-excitation), which in turn generates closely coupled premature beats as ventricular extrasystoles capable of precipitating PVT/VF23,24.
According to the depolarization theory, conduction slowing and delay, particularly in the right ventricular outflow tract (RVOT), results in delayed and abnormal depolarization currents that may play a primary role in the pathophysiology and arrhythmic manifestations of the syndrome22,23. Mild structural changes, such as increased collagen and fibrosis, and reduced expression of the gap junction protein connexin-43 especially (but not exclusively) in the RVOT, are a part of BrS and could account for the conduction abnormalities and also late potentials4. Further evidence supporting delayed depolarization includes the observation of late potentials and low-voltage fragmented electrogram activity in the RVOT and the RV anterior wall, and the beneficial effect of radiofrequency ablation on these epicardial sites of slow conduction in BrS patients4,25.
Despite the controversy, these two theories are not necessarily mutually exclusive and may indeed be synergistic5,26.
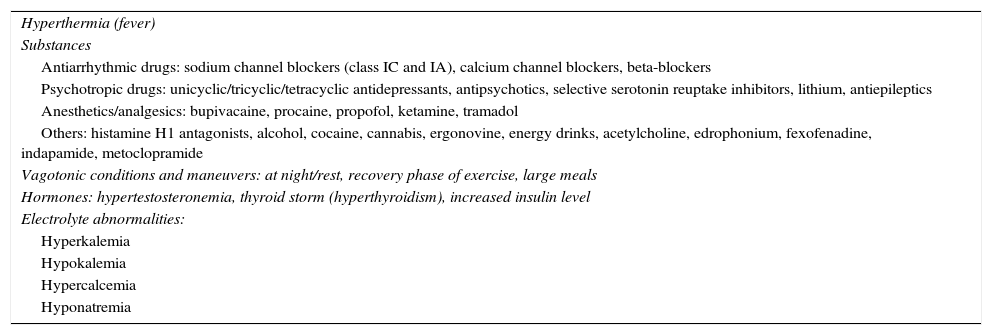
Agents and conditions associated with induced Brugada electrocardiographic patternCertain agents and conditions (Table 3) may act as modulating factors by influencing and interfering with cardiac ion channel function, unmasking the characteristic Brugada ECG pattern and possibly leading to fatal consequences, particularly in BrS patients, because of already impaired cardiac ion channel function3,5,16,17.
Agents and conditions modulating Brugada syndrome.
| Hyperthermia (fever) |
| Substances |
| Antiarrhythmic drugs: sodium channel blockers (class IC and IA), calcium channel blockers, beta-blockers |
| Psychotropic drugs: unicyclic/tricyclic/tetracyclic antidepressants, antipsychotics, selective serotonin reuptake inhibitors, lithium, antiepileptics |
| Anesthetics/analgesics: bupivacaine, procaine, propofol, ketamine, tramadol |
| Others: histamine H1 antagonists, alcohol, cocaine, cannabis, ergonovine, energy drinks, acetylcholine, edrophonium, fexofenadine, indapamide, metoclopramide |
| Vagotonic conditions and maneuvers: at night/rest, recovery phase of exercise, large meals |
| Hormones: hypertestosteronemia, thyroid storm (hyperthyroidism), increased insulin level |
| Electrolyte abnormalities: |
| Hyperkalemia |
| Hypokalemia |
| Hypercalcemia |
| Hyponatremia |
Fever is now recognized as being capable of unmasking BrS by promoting a type I Brugada ECG in susceptible individuals15,27–35. BrS is rarely identified in pediatric patients, but most reported cases are unmasked after febrile episodes29. A study by Adler et al.30 reported that type 1 Brugada ECG was 20 times more prevalent among patients with fever than in afebrile patients and that the prevalence of fever-induced BrS was 2%. Rattanawong et al.31 demonstrated an approximately five-fold higher prevalence of BrS in a febrile group compared to an afebrile group, with a prevalence of 4% in patients from endemic areas. Fever has also been reported to trigger VAs32–36. It was also demonstrated by Mizusawa et al.32 that asymptomatic patients with fever-induced type 1 ECG (F-type 1) carry a higher risk of arrhythmic events (0.9%/year), and conclude that patients with BrS who develop F-type 1 are at risk of arrhythmic events such as SCD. It has been observed that an SCN5A mutation identified in BrS patients leads to a loss of function of the sodium channel current that is accentuated at higher temperatures37. Compared with a drug challenge-induced type 1 pattern, an F-type 1 appears to have a more complex mechanism. Alternatively, other as yet unknown factors related to acute infection or increased temperature may be involved32. Nevertheless, fever is presumed to accelerate inactivation of INa and to accelerate recovery of Ito from inactivation5.
Similarly to fever, hypothermia may also induce or accentuate J waves, probably by slowing activation of ICa, leaving Ito unopposed5. However, it seems more likely to provoke Brugada-like ECG abnormalities, mimicking an actual BrS5,38,39. Moreover, the development of arrhythmias in BrS appears to be promoted only by fever, unlike early repolarization syndrome, in which hypothermia appears to induce VA5. It is noteworthy that hypothermia can even diminish the manifestation of a BrS ECG pattern when already present40.
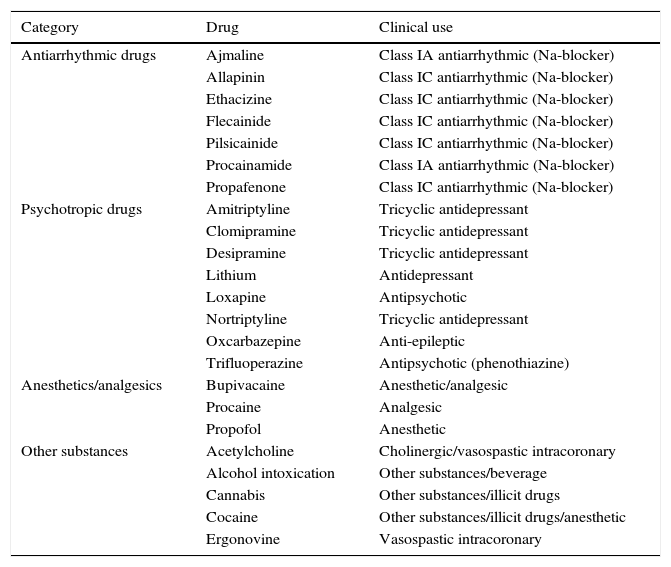
DrugsAn increasing number of drugs prescribed in routine clinical practice have been reported to induce or unmask the characteristic type 1 BrS pattern, predisposing to malignant VAs16–19. In view of the potential hazardous effects of certain drugs in BrS individuals, Postema et al.16 developed a website (www.brugadadrugs.org) with the goal of ensuring worldwide accessibility of information on safe drug use in BrS. Two broad categories of agents capable of unmasking a drug-induced type 1 (D-type 1) ECG were defined: one group that have a clear association with malignant VAs, to be avoided by BrS patients (Table 4), and another group, preferably to be avoided, for which there is as yet no substantial evidence that these drugs cause malignant arrhythmias as well as the D-type 1 phenotype (Table 5)16.
Agents to be avoided by Brugada syndrome patients (associated with type 1 ECG and related arrhythmias).
| Category | Drug | Clinical use |
|---|---|---|
| Antiarrhythmic drugs | Ajmaline | Class IA antiarrhythmic (Na-blocker) |
| Allapinin | Class IC antiarrhythmic (Na-blocker) | |
| Ethacizine | Class IC antiarrhythmic (Na-blocker) | |
| Flecainide | Class IC antiarrhythmic (Na-blocker) | |
| Pilsicainide | Class IC antiarrhythmic (Na-blocker) | |
| Procainamide | Class IA antiarrhythmic (Na-blocker) | |
| Propafenone | Class IC antiarrhythmic (Na-blocker) | |
| Psychotropic drugs | Amitriptyline | Tricyclic antidepressant |
| Clomipramine | Tricyclic antidepressant | |
| Desipramine | Tricyclic antidepressant | |
| Lithium | Antidepressant | |
| Loxapine | Antipsychotic | |
| Nortriptyline | Tricyclic antidepressant | |
| Oxcarbazepine | Anti-epileptic | |
| Trifluoperazine | Antipsychotic (phenothiazine) | |
| Anesthetics/analgesics | Bupivacaine | Anesthetic/analgesic |
| Procaine | Analgesic | |
| Propofol | Anesthetic | |
| Other substances | Acetylcholine | Cholinergic/vasospastic intracoronary |
| Alcohol intoxication | Other substances/beverage | |
| Cannabis | Other substances/illicit drugs | |
| Cocaine | Other substances/illicit drugs/anesthetic | |
| Ergonovine | Vasospastic intracoronary |
Adapted from www.brugadadrugs.org16 (last consulted on December 31, 2016).
Agents preferably avoided by Brugada syndrome patients (associated with type 1 ECG, but without substantial evidence that, apart from inducing the ECG phenotype, they also cause malignant arrhythmias).
| Category | Drug | Clinical use |
|---|---|---|
| Antiarrhythmic drugs | Amiodarone | Class III antiarrhythmic (also IA, II, and IV effects) |
| Cibenzoline | Class IA antiarrhythmic (Na-blocker) | |
| Disopyramide | Class IA antiarrhythmic (Na-blocker) | |
| Lidocaine | Class IB antiarrhythmic (Na-blocker) | |
| Propranolol | Class II antiarrhythmic (beta-blocker) | |
| Verapamil | Class IV antiarrhythmic (Ca-blocker) | |
| Vernakalant | Class I and III antiarrhythmic | |
| Psychotropic drugs | Bupropion | Monocyclic antidepressant |
| Carbamazepine | Anticonvulsant | |
| Clothiapine | Antipsychotic | |
| Cyamemazine | Antipsychotic (phenothiazine) | |
| Dosulepin | Tricyclic antidepressant | |
| Doxepin | Tricyclic antidepressant | |
| Fluoxetine | Antidepressant (SSRI) | |
| Fluvoxamine | Antidepressant (SSRI) | |
| Imipramine | Tricyclic antidepressant | |
| Lamotrigine | Anti-epileptic/bipolar and depressive disorders | |
| Maprotiline | Tricyclic antidepressant | |
| Paroxetine | Antidepressant (SSRI) | |
| Perphenazine | Antipsychotic (phenothiazine) | |
| Phenytoin | Anticonvulsant/antiarrhythmic | |
| Thioridazine | Antipsychotic | |
| Anesthetics/analgesics | Ketamine | Anesthetic |
| Tramadol | Narcotic analgesic | |
| Other substances | Dimenhydrinate | Antiemetic/histamine H1 antagonist |
| Diphenhydramine | Histamine H1 antagonist | |
| Edrophonium | Cholinergic | |
| Indapamide | Diuretic/antihypertensive | |
| Metoclopramide | Antiemetic/dopamine antagonist | |
| Terfenadine/Fexofenadine | Antihistamine |
SSRI: selective serotonin reuptake inhibitor.
Adapted from www.brugadadrugs.org16 (last consulted on December 31, 2016).
The mechanisms by which some of these drugs can induce a type 1 ECG and proarrhythmic effects are not yet fully clarified. However, most of them are either confirmed or believed to act through INa blockage, and a few may also act on other cardiac channels, by promoting a decrease in ICa or an increase in Ito currents18,19,41–56. The agents with INa blocking properties most strongly associated with a D-type 1 ECG and with clear evidence of proarrhythmic effects include ajmaline, flecainide, pilsicainide and propafenone (Table 1)16. Other antiarrhythmic agents include amiodarone which, although predominantly a potassium blocking agent (class III), also has INa blocking properties (as a class IA antiarrhythmic drug), especially in the acute phase of administration42. Vernakalant, which acts predominantly as a voltage- and rate-dependent INa blocker, has also recently been associated with Brugada ECG phenotype43.
Psychotropic and anesthetic drugs are among the non-cardiac drugs most commonly involved in drug-induced BrS19,41. Tricyclic antidepressants (TCAs) have been shown to produce a D-type 1 pattern and even VAs at therapeutic or supratherapeutic doses. TCAs, including amitriptyline, have been reported, among other actions, to block INa, thereby inducing the BrS phenotype. However, they can also potentially inhibit Ito, preventing its development18,19,44. Minoura et al.44 demonstrated that amitriptyline has a relatively potent inhibitory effect on INa and little inhibition of Ito at concentrations close to the therapeutic range, thus unmasking type 1 ECG and promoting arrhythmogenesis only in cases of a genetic predisposition. Similarly, blockage of INa by therapeutic as well as supratherapeutic dosages of nortriptyline has been demonstrated to be critical and cause life-threatening arrhythmias when other causes of INa block are also present, such as genetic variants and/or functional factors; the use of nortriptyline in the general population is associated with a 4.6-fold increased risk for SCA45. Lithium may also cause potent INa blockade in a dose-dependent manner, inducing a Brugada phenotype even at therapeutic dosages. Selective serotonin reuptake inhibitors at therapeutic or supratherapeutic doses have been implicated in D-type 1 ECG without associated VA, probably by INa blockade. As well as supratherapeutic doses of certain antipsychotics causing a D-type 1 pattern, therapeutic doses of loxapine and trifluoperazine have been associated with VF18,19,41. Some antiepileptic agents, including lamotrigine, are also capable of inducing a D-type 1 pattern, presumably acting on cardiac INa as well as the sodium channels in the cerebral cortex19,46.
Propofol is a widely used anesthetic agent with few significant side effects. However, at high doses, it may be associated with SCD, in a condition termed propofol infusion syndrome, whose mechanism of arrhythmogenesis is thought to be similar to that responsible for VA in BrS, probably by blocking INa18,19,41,47. Procaine, which produces anesthesia by sodium channel blockade, was recently reported to have unmasked BrS and contributed to SCA in a young male individual48. Tramadol is a commonly prescribed synthetic opioid analgesic that in overdose may produce INa blockade and a D-type 1 pattern49.
A D-type 1 ECG has also been elicited in patients treated with antihistamines (mainly first-generation). No associated VAs have been reported with their use in isolation18,19. Leiria et al.50 described an interesting case of a 44-year-old man in whom BrS was diagnosed after experiencing a syncopal episode associated with the use of over-the-counter cold medicine (brompheniramine + phenylephrine). The mechanism by which adrenergic agonists can trigger BrS is not entirely understood, but one hypothesis is a sudden increase in vagal tone once the adrenergic effect has worn off. Alternatively, brompheniramine appears to directly alter the expression of the SCN5A gene and to reduce the INa current50. Alcohol intoxication has been shown to induce the Brugada ECG pattern along with VA. The exact mechanism is not clear, but increased parasympathetic nervous activity and inhibition of ICa currents have been proposed as possible underlying causes19,51. Similarly, acetylcholine and ergonovine have also been reported to decrease ICa currents and possibly to induce VF in BrS patients52. In addition to sympathomimetic actions that can result in acute ischemic events, cocaine has a potent direct blocking effect on INa that, particularly at relatively low doses, can trigger VF in genetically predisposed individuals with BrS19,41,53. Cannabis has also been reported to be associated with the Brugada pattern. The mechanisms of this interaction are unclear, but may be related to a late vagotonic effect after cannabis exposure54,55. The Red Bull® energy drink was recently reported to have induced SCA with VF in a young individual who was eventually diagnosed with BrS. Red Bull contains taurine, which could suppress INa, ICa and Ito channels, and high levels of caffeine, which could disrupt calcium homeostasis and lead to cytoplasmic calcium overload, thus potentiating VA56. Several antianginal drugs may also be associated with a D-type 1 pattern, but evidence is lacking on the existence and nature of this relationship16,18,57.
Vagotonic conditions and maneuversVagal tone is recognized as an important factor capable of precipitating VAs in BrS, which explains why VF and SCD are more frequent at night, at rest, and at low levels of physical activity9,12–14, since these situations are related to higher levels of vagal tone and slower heart rates. Specifically, vagal tone is thought to directly inhibit ICa and indirectly increase Ito due to slowing of heart rate. This autonomic modulation of ion channel currents underlying the early phases of the epicardial AP may therefore contribute to the characteristically dynamic ECG in BrS5,9,12–14.
Large meals have similarly been implicated in unmasking BrS and precipitating VA in BrS patients, some even going so far as to propose that they could play a role in the diagnosis of BrS in suspected cases21,58,59. Velázquez-Rodríguez et al.58 recently demonstrated the reproducibility, efficacy and safety of the dextrose-insulin metabolic test in the differential diagnosis of patients with non-diagnostic ECG patterns. The spontaneous J wave and ST-segment elevation seen after meals may reflect changes in autonomic modulation due to a full stomach, with consequent increase in vagal activity, and may also be a consequence of high glucose concentrations and insulin release58,59.
The increase in vagal tone that occurs immediately after exercise appears to be the reason that the Brugada pattern can be seen in BrS patients with exercise, especially early in the recovery phase. Besides worsening ST-segment elevation in BrS, exercise can produce associated VAs. Exercise testing may also be helpful in unmasking BrS14,60,61.
HormonesApart from insulin as mentioned above, other hormones may also play a role in the manifestation of the Brugada ECG pattern, associated VA and BrS itself. Testosterone is thought to modulate ion currents underlying the epicardial AP notch, possibly by promoting an increase in Ito or a decrease in ICa currents5,62,63. In addition to the presence of a more prominent Ito current in males, higher testosterone levels associated with less visceral fat also appears to have a significant role in the Brugada phenotype and in the male predominance in BrS62,63. Moreover, thyroxine may alter membrane currents, including Ito and ICa, thus contributing to triggering the Brugada pattern and BrS62,64. Recently, Korte et al.64 reported a case of SCA as a presentation of BrS unmasked by a thyroid storm in a young male.
Electrolyte imbalanceIt has been speculated that certain electrolyte disturbances can amplify the Ito-mediated AP notch with loss of the AP dome in the epicardium of the RVOT, thereby precipitating the Brugada ECG pattern, and even related VAs in BrS patients3,5,36,65.
Hyperkalemia may induce the Brugada pattern by decreasing the resting membrane potential, which inactivates the INa current and leads to a predominantly Ito current that is most pronounced in the RV epicardium, resulting in type 1 Brugada ECG24,65. Postema et al.66 also reported a case of diabetic ketoacidosis with concomitant hyperkalemia that uncovered a typical Brugada pattern, and further pharmacologic challenge in the patient and his son confirmed familial BrS. The concomitant acidosis may also have played an important part because of its similar effect in decreasing INa currents66,67.
Hypokalemia is also known to accentuate the Brugada ECG pattern, possibly by enhancing Ito with an increase in transmural or epicardial dispersion of repolarization in the RV, which may increase the risk for VF in patients with BrS57,68.
Furthermore, hypercalcemia may also unmask the Brugada-type ECG, probably through transmural differences in the magnitude of the AP notch due to an increase in the calcium-activated chloride current and to a reduction of INa and ICa69,70. However, it is unknown whether hypercalcemia-induced J-point elevation increases the risk of VA70.
Hyponatremia is believed to diminish the ion gradient and thereby reduce the INa current, leaving Ito unopposed, which may cause loss of the AP dome in the RV epicardium. However, whether induction of the Brugada pattern by severe hyponatremia is associated with increased susceptibility to VA is currently uncertain71.
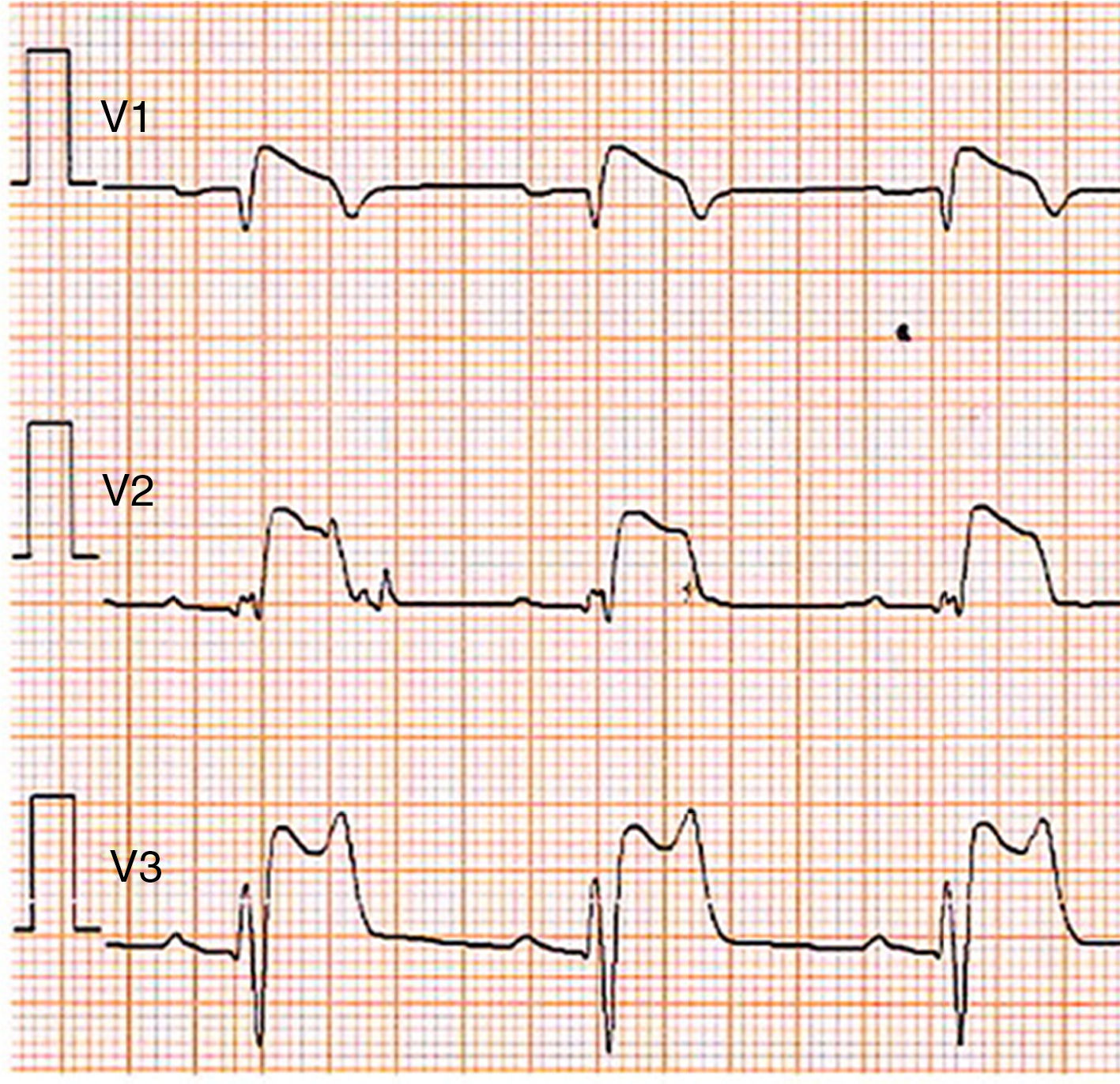
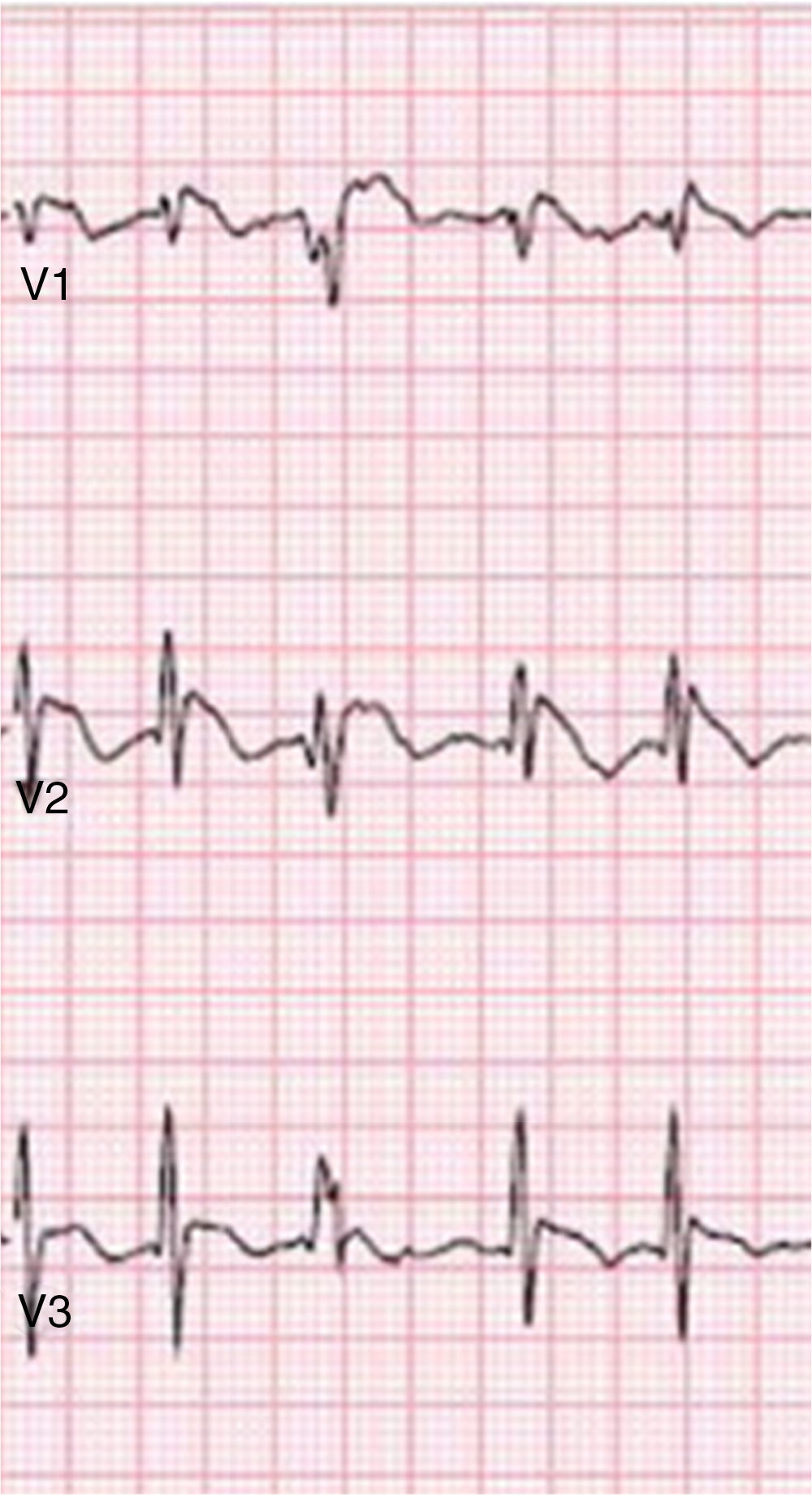
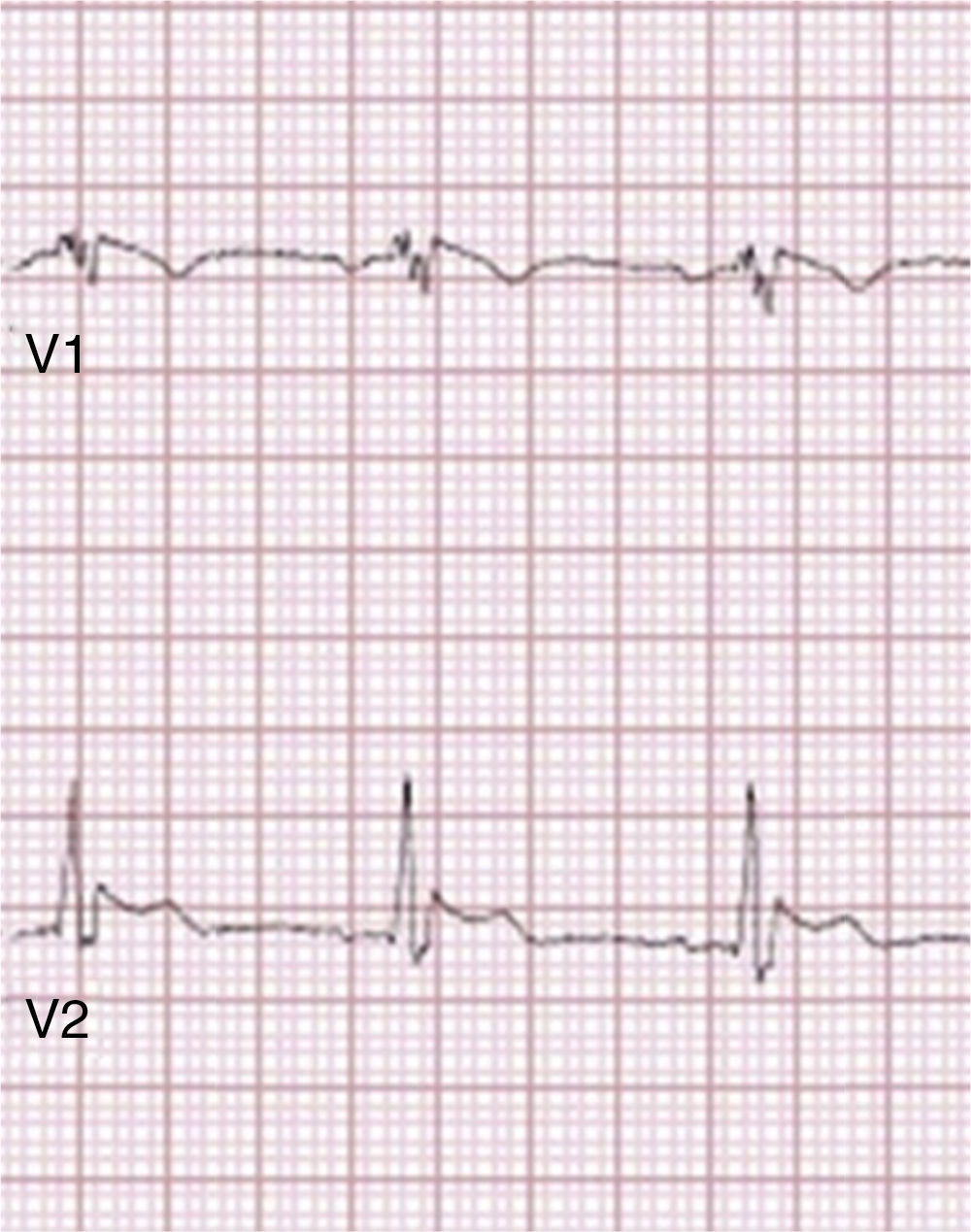
Brugada phenocopiesBrPs are clinical entities that have visually similar or even identical ECG patterns to true congenital BrS but are etiologically distinct, being elicited by a variety of other clinical circumstances57,72,73. The term phenocopy describes a phenotype which is caused by environmental conditions that matches one determined by a gene, and so the absence of any apparent genetic abnormality is central to the concept of BrP; the environmental factors alone are sufficient to result in a BrS pattern rather than unmasking latent BrS57,73–75. These factors may include myocardial ischemia, hyponatremia, hyperkalemia (Figure 2), hypokalemia, hypophosphatemia (Figure 3), pulmonary embolism, concomitant alcohol and heroin overdose, hypothermia, hypopituitarism, mechanical mediastinal compression, electrocution (Figure 4), and poor ECG filters; they are categorized by etiology in Table 638,73–87. The website www.brugadaphenocopy.com was recently created to establish an online international database of BrP cases to allow for longitudinal follow-up of these conditions and to develop a better understanding of BrP74. Notably, a recent report of recurrent hypokalemia demonstrated clinically reproducible BrP, contributing to the evolution of the concept79.
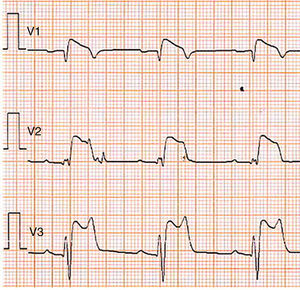
Type 1 Brugada phenocopy electrocardiogram in V1-V2 and type 2 Brugada phenocopy electrocardiogram in V3, in the context of hyperkalemia. Retrieved from Dendramis et al.78.
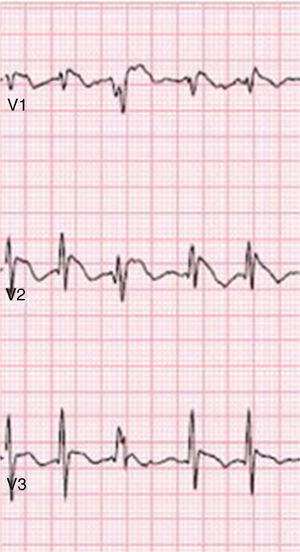
Type 1 Brugada phenocopy electrocardiogram in V1-V3, in the context of hypophosphatemia. Retrieved from Meloche et al.80.
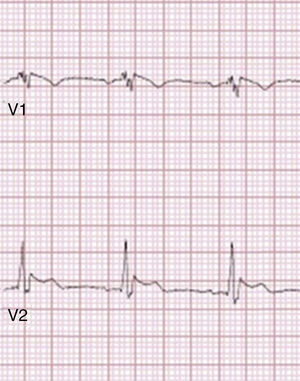
Type 1 Brugada phenocopy electrocardiogram in V1 and type 2 Brugada phenocopy ECG in V2, in the context of electrocution. Retrieved from Wang et al.85.
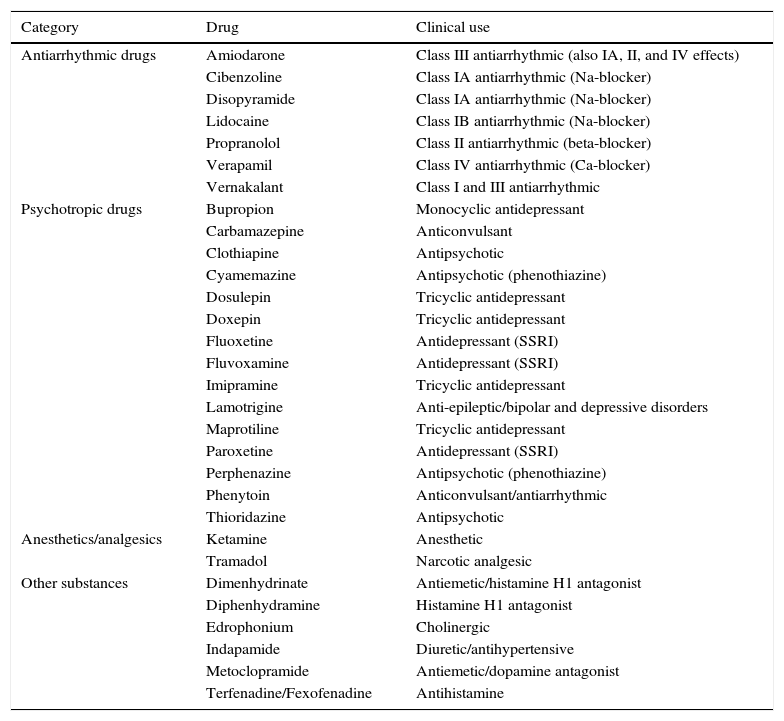
Possible etiological categories of Brugada phenocopies.
| Metabolic conditions |
| Mechanical compression |
| Ischemia and pulmonary embolism |
| Myocardial and pericardial disease |
| ECG modulation |
| Miscellaneous |
Adapted from Anselm et al.86
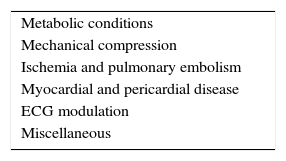
In order to differentiate BrP from true congenital BrS, the criteria summarized in Table 7 can be used to assist with recognition and diagnosis of BrP. Unlike in BrS, in BrP there is usually an identifiable and reversible underlying condition, resolution of which is accompanied by prompt normalization of the ECG. Additionally, these patients have low clinical probability for BrS, as indicated by the absence of a documented personal history of SCA or syncope, or family history of SCD. Finally, drug challenge testing is negative, while in BrS patients this tends to be positive73,74. The differences in ECG response to drug challenge denote pathophysiological differences between BrP and BrS, suggesting alternative underlying mechanisms with various genetic, structural and environmental interactions that are yet to be elucidated73. While high-risk patients with BrS may be candidates for an implantable cardioverter-defibrillator (ICD), the clinical implications of BrP, and hence the correlation between BrP and malignant VA, remain unknown5,57,73. At this time, the recommended approach for BrP is mainly based on systematic diagnostic procedures (to differentiate BrP from BrS) and resolution of the underlying condition73,74.
Diagnostic criteria for Brugada phenocopy.
| 1. The ECG pattern has a type 1 or type 2 Brugada morphology |
| 2. The patient has an underlying condition that is identifiable |
| 3. The ECG pattern resolves after resolution of the underlying condition |
| 4. There is a low clinical pretest probability of true BrS determined by lack of symptoms, medical history, and family history |
| 5. The results of drug challenge with a sodium channel blocker such as ajmaline, flecainide, pilsicainide or procainamide are negative |
| 6. The results of genetic testing are negative (desirable but not mandatory, because an SCN5A mutation is identifiable in only 18-28% of probands affected by true BrS) |
BrS: Brugada syndrome; ECG: electrocardiographic.
Adapted from Anselm et al.87
Criteria 1-5 are mandatory, except for criterion 5 if there was surgical manipulation of the right ventricular outflow tract within 96hours of the presenting Brugada ECG pattern.
The congenital BrS ECG pattern is often dynamic and concealed, but it can be unmasked, modulated or precipitated by a wide range of agents and conditions, which are summarized in Table 33,5,8. Associated malignant VAs can develop under most of these circumstances in BrS patients, because these individuals already have impaired cardiac ion channel function3,5,17. This association with VA and SCD is more evident with fever15,32–36, with certain drugs that are indicated to be avoided by BrS patients (Table 4)16 and energy drinks56, under vagotonic conditions9,12,13 such as a large meal21,58,59 or the recovery phase of exercise14,60,61, with hypertestosteronemia62,63 or hyperthyroidism64, and possibly with potassium imbalance65–68. Fever is recognized as a major risk factor in BrS and can even outperform drug challenge in unmasking a type 1 ECG5,28,32. Junttila et al.36 reported that patients presenting with a Brugada ECG during an acute event, including fever, treatment with medication affecting INa, drug overdose or electrolyte imbalances, should be considered to have a higher risk of life-threatening VA and SCD, even in the absence of an SCN5A mutation. It is also plausible that the cardiac electrophysiological mechanisms involved in acidosis and ischemic heart disease may potentiate those of BrS, resulting in increased risk for VA in BrS patients52,67,88. In most of such cases, the type 1 pattern and associated VAs presumably result from induced or exacerbated decrease in inward currents (INa and ICa) or increase in outward currents (Ito or IK-ATP)3,5,20. There is now a growing consensus that apart from genetic factors, the expression of BrS is also multifactorial, the underlying genetic predisposition being modulated by diverse environmental factors and even morphologic cardiac changes4,5.
Equally important, there are also cases of acquired forms of BrS in which, theoretically, an intervention (such as administration of a pharmacologic agent) could have caused a sufficient imbalance of inward and outward currents in the RVOT to induce a Brugada pattern17–20,75. These patients are typically asymptomatic, without a personal or family history of VA, and may test positive on drug challenge. It is not yet established whether this requires an underlying genetic predisposition or actually represents latent BrS18,75. However, the susceptibility of these individuals may be due to genetic polymorphisms rather than pathogenic mutations17,75,89. Such a genetic predisposition or subclinical form (forme fruste) may result in increased latent ion channel dysfunction that favors induction of a Brugada pattern, particularly when there is a specific combination of stressors and comorbidities, similar to what is observed with acquired long QT syndrome (LQTS)17,18,75,90. However, the likelihood of arrhythmias is still unclear18,75.
In principle, a risk profile for a D-type 1 pattern and VA could be drawn up based on various factors such as male gender, genetic predisposition and modulating polymorphisms, specific electrolyte disturbances, fever, kidney and liver dysfunction, cardiac comorbidities, the use or excessive doses of specific drugs, electrocardiographic characteristics, and previous arrhythmias17,91. Konigstein et al.92 recently documented that the D-type 1 ECG associated with non-cardiac drugs is seen mostly in adult males, is frequently due to drug toxicity, and develops late after the onset of therapy. However, it would be difficult to apply these data to a specific individual, because of the wide variability in response to a specific drug, and because its possible effects may change over time depending on treatment duration, age, or developing comorbidities. Furthermore, most of the evidence supporting the association with VAs comes from a limited number of case reports, laboratory studies and cohort analyses16,17. It would therefore be difficult to predict the likelihood of drug-induced BrS in routine clinical practice17,19,91.
Clinical presentation is still the strongest predictor of risk in BrS, and in asymptomatic patients the arrhythmic risk is low (0.5% per year). Furthermore, asymptomatic patients with a type 1 pattern that only develops following drug challenge have a lower risk of arrhythmic events during follow-up than those with a spontaneous type 1 at diagnosis5,93. Unlike high-risk BrS patients, who may have indication for an ICD, asymptomatic patients with a type 1 pattern only disclosed by drug challenge may be indicated for close follow-up only5,6. The prognosis of asymptomatic patients with a D-type 1 pattern but without a family history of SCD appears to be relatively benign once the offending agent is discontinued5,18. Nevertheless, it may be prudent not to ignore the risk, particularly during exposure to the agent16–19,36,41,91.
There is considerable debate concerning the appropriate terminology for BrP, mainly because it is very difficult to rule out a genetic predisposition. Antzelevitch et al. have accordingly proposed that these conditions be designated acquired forms of Brugada ECG pattern or BrS, as more in line with the terminology used in LQTS.5 At this stage, there is a need to clarify the concepts and real significance of acquired Brugada patterns and BrP as clinical entities, especially in terms of arrhythmic risks and prognosis, and to gain a better understanding of their underlying mechanisms and the possible genetic predisposition in the case of acquired BrS. Thus, more systematic, population-based, observational and experimental studies are needed to clarify these concepts and to better differentiate them from each other and from BrS itself.
Nevertheless, in a patient with an induced type 1 Brugada pattern, it seems prudent to proceed with a systematic diagnostic approach, excluding confounding factors (Table 2), identifying possible underlying modulators (Table 3), investigating whether the ECG normalizes on resolution of possible environmental factors, investigating personal and family history of symptoms and arrhythmias (including SCD), performing a drug challenge (Table 1), and possibly undertaking genetic study, including of the patient's family5,8,19,73. Additionally, distinguishing true BrS from BrP is crucial, because the type 1 pattern in BrS can be transient and indistinguishable from BrP and possibly provoked by the same conditions, such as hyperkalemia66,72–74,78. Moreover, the propensity for VA in BrS is established, whereas this is not the case for BrP73,74. Since the prognosis of BrP may be related to the evolution of the underlying condition73,74, and a type 1 Brugada ECG may itself be an indicator of increased risk for arrhythmias17, it makes sense to screen for and promptly correct any underlying conditions or possible modulating factors5,73,74. Fever in BrS patients should be treated aggressively with antipyretics, and they should avoid the substances listed in Tables 4 and 5 and be familiar with the effects of a large meal, especially after a long fasting period5,16–19,59. Data on the risks of exercise in BrS are currently too limited to make recommendations concerning exercise5,60. However, it is suggested that BrS patients should avoid vigorous exercise60,61.