The authors present a rare case of hypertrophic cardiomyopathy associated with left ventricular noncompaction cardiomyopathy and coronary artery-left ventricular fistulae in a 42-year-old woman presenting with non-ST-elevation myocardial infarction. Coronary angiography, transthoracic echocardiography and cardiac magnetic resonance revealed the structural abnormalities of the left ventricle and the coronary tree.
Os autores apresentam um raro caso de miocardiopatia hipertrófica (MCH) associada a ventrículo esquerdo não compactado (VENC) e fístulas das artérias coronárias para o ventrículo esquerdo (VE) numa doente de 42 anos, que se apresenta com enfarte agudo do miocárdio sem elevação do segmento ST (EAMSEST). A coronariografia, a ecocardiografia transtorácica (ETT) e ressonância magnética cardíaca (RMC) revelaram as alterações estruturais do VE e da árvore coronária.
Hypertrophic cardiomyopathy (HCM) is the most common genetic heart disease, with a prevalence of 1:500.1 Although it is phenotypically varied,2,3 it is characterized by the presence of left ventricular (LV) hypertrophy with preserved systolic function and diminished relaxation, in the absence of other identifiable causes.4 Inheritance is autosomal dominant, and over 900 mutations have been reported in at least 11 genes coding for sarcomere proteins.5 The most frequent mutations involve the beta-myosin heavy chain (MYH7), cardiac troponin T (TNNT2) and cardiac myosin-binding C protein.6
Histopathological study reveals myocardial disarray and fibrosis.4
Clinical diagnosis is difficult, particularly with non-obstructive forms. The classical echocardiographic criteria for a diagnosis of HCM are maximum wall thickness of >15 mm in any myocardial segment, with predominantly asymmetrical septal involvement; normal global systolic function with diastolic dysfunction; intraventricular gradient; mitral systolic anterior motion and regurgitation; and granular appearance of the myocardium.2,4,7,8 Assessment of the extent and severity of hypertrophy should include measurement of maximum wall thickness in several segments. Clinical diagnosis may be supported by other typical characteristics, including family history of heart disease, exertional dyspnea, angina, syncope, tachyarrhythmias, mitral regurgitation and electrocardiographic alterations.9 Genetic study can be used to screen relatives for mutations, but does not predict the clinical course of a given patient, although this may be influenced by particular mutations.6,10
Left ventricular noncompaction (LVNC) is a rare cardiomyopathy, usually genetic but sometimes acquired,11 with a prevalence of less than 1:5000.12 One theory for its origin is that the process of myocardial compaction is arrested during embryogenesis. Around the end of the fourth week of gestation, endocardial invaginations begin to cross the embryonic jelly to the adjacent myocardium, in which sinusoidal recesses develop, which subsequently become part of the coronary circulation.13 The lacy pattern of trabeculations protruding into the ventricular lumen give the heart a spongy appearance. Between the 12th and 18 weeks of gestation, the myocardium undergoes a gradual process of compaction, from the base of the heart to the apex,12,14 mediated by various neurohumoral and angiogenic growth factors.15
There are two types of LVNC: those without and those with other congenital heart defects, including coronary microfistulae.16 Morphological features on echocardiography include excessive LV trabeculations, with a ratio of ≥2:1 between non-compacted and compacted layers at end-systole, and endomyocardial recesses communicating with the ventricular cavity.17 LVNC mainly affects the LV apex and mid and distal posterolateral segments.18 These segments are typically hypokinetic, resulting in systolic dysfunction.17 Mutations in genes coding for sarcomere proteins that had previously been linked to the pathogenesis of HCM have recently been identified in LVNC patients.19,20 Symptoms are nonspecific, ranging from heart failure and conduction disturbances to embolic events due to thrombus formation in intertrabecular recesses.15 The heterogeneous nature of this cardiomyopathy means that prognosis is variable.21
Case reportWe present the case of a 42-year-old woman admitted to the emergency department with chest discomfort, palpitations and dyspnea for the previous two hours. She had a history of HCM, permanent atrial fibrillation (AF) and stroke in 2002, at which time transthoracic echocardiography revealed a hypertrophied ventricular septum (around 18 mm) with no intraventricular gradient, dilated left atrium (42 mm), preserved global left ventricular function and no images suggestive of intracardiac thrombi. She was now medicated with carvedilol 6.25 mg twice daily, digoxin 0.25 mg daily and warfarin for a target INR of 2–3. She had a family history of HCM with a 40-year-old brother affected and a brother and a sister suffering sudden cardiac death at the ages of 24 and 46, respectively.
Physical examination revealed no abnormalities apart from arrhythmia on cardiac auscultation. The electrocardiogram showed AF with controlled ventricular response and nonspecific intraventricular conduction disturbances. The chest X-ray revealed cardiomegaly without pulmonary edema. Laboratory test results included elevated troponin I (5.2 ng/ml) and hypercholesterolemia.
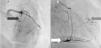
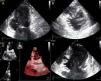
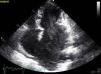
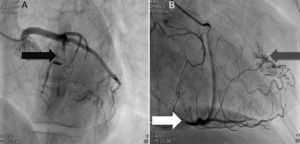
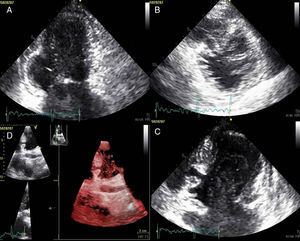
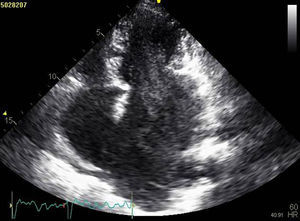
The patient was admitted with a diagnosis of non-ST-elevation myocardial infarction. Cardiac catheterization performed to exclude coronary disease identified myocardial bridging at various points along the left coronary artery (mid anterior descending, septal branches and distal branches of the first diagonal), as well as several left and right coronary artery-left ventricular fistulae. A recanalized thrombus was observed in the posterolateral branch. Two-dimensional transthoracic echocardiography showed asymmetric LV hypertrophy mainly involving the ventricular septum (22 mm) and lateral walls and a basal posteroinferior aneurysm. Global systolic function was preserved (∼55% by Simpson's biplane method). Two intramyocardial recesses were observed in the basal part of the inferoseptal wall communicating with the LV cavity, around 4 mm in diameter, the larger one traversing almost the entire thickness of the wall but with no interventricular communication. The left atrium was severely dilated and spontaneous contrast was seen in the left chambers.
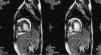
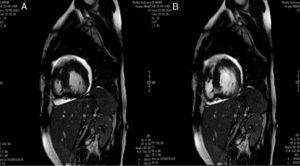
Cardiac magnetic resonance imaging performed to confirm the diagnosis revealed a ratio of 2.7:1 of non-compacted to compacted layers, as well as images suggestive of intramural recesses, and preserved LV systolic function, as previously shown by transthoracic echocardiography.
Genetic study identified the c.1208G>A mutation in exon 13 of the MYH7 gene.
The patient was discharged after 12 days, medicated with metoprolol 50 mg twice daily, atorvastatin 10 mg daily, warfarin and furosemide 40 mg daily.
One month later, an implantable cardioverter-defibrillator was implanted. Apart from a brief hospitalization for decompensated heart failure, the patient has remained stable.
DiscussionThe authors present a rare case that combines the phenotype of two cardiomyopathies, HCM and LVNC, together with multiple coronary artery-ventricular fistulae and large intramyocardial, probably sinusoidal, recesses.
We presume that the simultaneous presence of coronary fistulae, intramyocardial recesses and spongy myocardium results from an abnormality in embryonic development, in which defective regression of the sinusoids and myocardial compaction resulted in a single disease.22–24 The compacted layer should give rise to the ventricular myocardium, but arrested compaction results in persistence of intertrabecular recesses and incomplete formation of capillaries.13,17
Unlike arteriovenous or arterio-arterial fistulae, coronary artery-ventricular fistulae are extremely rare. They are more common in females and most are congenital.25 Their clinical significance depends on their size and location; most are asymptomatic and incidental findings.26
Multiple coronary artery-left ventricular fistulae have occasionally been detected in cases of apical HCM.27–29 They may be associated with regional hypertrophy, although it is not clear whether this is the cause or the effect of anomalies of coronary anatomy.27 The mechanisms of myocardial ischemia in HCM include increased oxygen demand by the hypertrophied ventricular myocardium, impaired coronary circulation and increased diastolic filling pressures. Coronary fistulae and resulting coronary steal and left-to-left shunt reduce ventricular perfusion and increase diastolic volume overload.23,29 In the case presented, despite the large number of fistulae, LV systolic function was preserved.
Studies have shown that the presence of a single mutation in a gene coding for a sarcomere protein, such as MYH7 Arg243His, can lead to different patterns of pathological myocardial remodeling, particularly HCM and LVNC. These variations in phenotypic expression may reflect interactions between genotype and environmental factors.20,30 The mutation identified in the family in this family (Arg403Gln) is associated with HCM but not LVNC20; an estimated 50% of carriers of this mutation suffer sudden cardiac death before the age of 50.31 An interesting aspect of this case is the fact that a surviving brother of the patient has the same mutation, but has not developed LVNC or coronary artery-ventricular fistulae, which supports the idea that the patient's two cardiomyopathies have different etiologies, i.e. that the HCM is of genetic origin and the LVNC was due to embryonic arrest.
We were unable to determine the etiology of the posteroinferior aneurysm observed on transthoracic echocardiography and previous exams did not clarify at what point it developed.
Systemic thromboembolism is a complication of cardiomyopathies, especially when associated with left atrial dilatation and AF.32 The source of systemic emboli associated with HCM is intracardiac thrombi caused by blood pooling in the dilated atrium or in ventricular aneurysms.33 The incidence of thromboembolic events in LVNC patients ranges between 5% and 38%.34
The mechanisms leading to coronary thrombosis in individuals with coronary fistulae include abnormal flow patterns associated with vascular ectasia, coronary tortuosity, and abrupt changes in vessel caliber; intrinsic changes in the clotting or fibrinolytic cascade; and effects of direct vascular trauma .35
In the case presented, there are three possible causes for the patient's non-ST-elevation myocardial infarction: (1) an embolic event due to AF; (2) intracoronary thrombosis associated with coronary fistulae, probably due to the phenomena described above; and (3) coronary bridging due to reduced coronary perfusion caused by relaxation delay of the arterial wall at beginning of diastole. The patient's history of ischemic stroke shows that she is at high risk for further thromboembolic events.
To our knowledge, the case presented is the first diagnosed during life of the combination of two cardiomyopathies and multiple coronary artery-ventricular fistulae. It highlights the complementary role of different imaging techniques for thorough assessment of such cardiovascular anomalies (Figures 1–4).
Coronary angiography showing (A) bridging at various points along the left coronary artery (black arrow: anterior descending) and (B) several right and left coronary artery-left ventricular fistulae (gray arrow). A recanalized thrombus can be seen in the posterolateral branch (white arrow).
Transthoracic echocardiography showing (A) basal posteroinferior aneurysm (two-dimensional apical 2-chamber view); (B) endomyocardial trabeculations and recesses communicating with the ventricular cavity (two-dimensional parasternal short-axis view); (C and D) two intramyocardial recesses in the basal portion of the inferoseptal wall communicating with the left ventricular cavity, around 4 mm in diameter, the larger one traversing almost the entire thickness of the wall but with no interventricular communication (two- and three-dimensional images, respectively, apical 4-chamber view).
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Please cite this article as: Delgado A, Moreira D, Rodrigues B, et al. Miocardiopatia hipertrófica associada a ventrículo esquerdo não compactado e fístulas coronárias – a propósito de um caso clínico. Um genótipo, três fenótipos? Rev Port Cardiol. 2013. http://dx.doi.org/10.1016/j.repc.2013.05.002