Highly active antiretroviral therapy (HAART) has created a new paradigm for human immunodeficiency virus (HIV)-infected patients, but their increased risk for coronary disease is well documented.
We present the case of a 57-year-old man, co-infected with HIV-2 and hepatitis B virus, adequately controlled and with insulin-treated type 2 diabetes and dyslipidemia, who was admitted with non-ST elevation acute myocardial infarction. Coronary angiography performed on day four of hospital stay documented two-vessel disease (mid segment of the right coronary artery [RCA, 90% stenosis] and the first marginal). Two drug-eluting stents were successfully implanted. The patient was discharged under dual antiplatelet therapy (aspirin 100 mg/day and clopidogrel 75 mg/day) and standard coronary artery disease medication. He was admitted to the emergency room four hours after discharge with chest pain radiating to the left arm and inferior ST-segment elevation myocardial infarction was diagnosed. Coronary angiography was performed within one hour and documented thrombosis of both stents. Optical coherence tomography revealed good apposition of the stent in the RCA, with intrastent thrombus. Angioplasty was performed, with a good outcome.
The acute stent thrombosis might be explained by the thrombotic potential of HIV infection and diabetes. There are no specific guidelines regarding HAART in secondary prevention of acute coronary syndromes. A multidisciplinary approach is essential for optimal management of these patients.
A terapêutica antiretroviral (TARV) alterou o paradigma da infeção pelo vírus da imunodeficiência humana (VIH), conhecendo-se o risco aumentado de doença coronária nestes doentes.
Apresenta-se o caso de um homem de 57 anos, melanodérmico, com coinfecção VIH-2/vírus hepatite B, com controlo adequado; diabetes mellitus tipo 2, insulino-tratado e dislipidemia. Internado por enfarte agudo do miocárdio, sem supradesnivelamento ST. Realizou cateterismo ao 4.° dia de internamento, documentando-se doença de dois vasos (segmento médio da coronária direita [CD] [90% estenose] e 1.ª obtusa marginal [OM1] com estenose de 95%). Colocaram-se dois stents revestidos, sem intercorrências. Teve alta sob dupla antiagregação (ácido acetilsalicílico 100 mg/dia e clopidogrel 75 mg/dia) e restante terapêutica dirigida à doença coronária. Recorreu ao serviço de urgência quatro horas após a alta por pré-cordialgia com irradiação ao membro superior esquerdo, tendo-se diagnosticado enfarte agudo do miocárdio com supradesnivelamento do segmento ST nas derivações inferiores. Realizou coronariografia uma hora após o início da dor, que revelou oclusão de ambos os stents. A tomografia de coerência ótica (OCT) revelou boa aposição do stent na CD, trombos intra-stent e dissecção com início na margem distal do stent. Realizou-se angioplastia de ambas as artérias, com sucesso.
A trombose aguda dos stents pode ser explicada pelo aumento do potencial trombótico conferido pelo VIH e pela diabetes. Não existem recomendações específicas relativas à TARV na prevenção secundária após SCA. A abordagem multidisciplinar destes doentes é essencial para a sua orientação adequada.
The advent of highly active antiretroviral therapy (HAART) has led to a marked decrease in morbidity and mortality among patients with human immunodeficiency virus (HIV) infection,1–4 and a consequent increase in their life expectancy and in the prevalence of chronic diseases not previously associated with HIV. These include cardiovascular and liver disease and cancer,5–7 the treatment of which is becoming increasingly important in these patients.
There is in fact a well-documented higher risk of coronary artery disease (CAD) in HIV patients compared to the general population.3,8,9 In a study carried out by the Antiretroviral Therapy Cohort Collaboration,6 which included 39272 patients from 13 cohorts of patients receiving HAART between 1996 and 2006 in Europe and the USA, mortality attributed to cardiovascular disease in HIV patients was 7.9% (40% of which was due to CAD or myocardial infarction [MI] and 18% to stroke). The D:A:D study,7 which was completed later and included 49731 patients followed between 1999 and 2011 in clinics in Europe, the USA and Australia, reported mortality of 11% due to cardiovascular disease.
There are few data on the risk of adverse cardiovascular events in HIV-infected patients undergoing percutaneous coronary intervention (PCI) with drug-eluting stents in the context of acute coronary syndrome (ACS). Some studies10–12 have reported similar rates for infected and non-infected patients.
We report the case of an HIV patient with diabetes who presented acute thrombosis of drug-eluting stents in two different coronary arteries four hours after hospital discharge, which manifested as ST-elevation MI.
An unusual case of acute stent thrombosis in an HIV patient is presented, together with a literature review and discussion of possible explanations for this atypical presentation.
Case reportA 57-year-old man, black, born in Guinea and resident in Portugal since 1982, an astrologer, had a personal history of co-infection with HIV-2 and hepatitis B virus (HBV). HIV-2 was diagnosed in 1997 (19 years before this hospitalization), when HAART was begun. The patient's CD4+ T cell count before therapy is unknown. He was regularly monitored and complied with therapy, which included the antiretrovirals lamivudine, stavudine, indinavir and abacavir.
The patient's viral load and CD4+ T cell count were generally under control from the time of diagnosis, except for a period of one year approximately two years before this admission, when he presented an increased viral load (maximum 4500 copies/ml, although his CD4+ T cell count was controlled throughout this period).
At admission, the patient was under combined HAART, namely tenofovir and emtricitabine 300 mg/200 mg once daily; darunavir in combination with ritonavir 600 mg/100 mg twice daily and raltegravir 400 mg twice daily. He presented adequate CD4+ T cell counts and negative viral load. HBV infection was diagnosed in 2010 (six years before this hospitalization), and regular monitoring continued to show negative viral load under tenofovir therapy.
He had a history of insulin-treated type 2 diabetes, diagnosed 17 years prior to this admission, with no known microvascular complications, and mixed dyslipidemia (predominantly hypertriglyceridemia), diagnosed 10 years previously. Laboratory tests at the time of diagnosis showed total cholesterol 205 mg/dl, high density lipoprotein (HDL) cholesterol 21 mg/dl, low density lipoprotein (LDL) cholesterol 90 mg/dl, and triglycerides 265 mg/dl. Therapy with fenofibrate 145 mg/daily was begun, which the patient had continued ever since. Persistently high total and LDL cholesterol levels during follow-up led to atorvastatin 20 mg being added around four years previously.
The patient had no other risk factors, including smoking, cocaine use, hypertension, obesity or family history of sudden death.
Besides HAART, he was thus being medicated as an outpatient with atorvastatin 20 mg, fenofibrate 145 mg, pantoprazole 20 mg, linagliptin 5 mg and insulin (Insulatard®).
The patient went to the emergency department (ED) due to right chest pain radiating to the neck that had begun that morning as soon as he arose, not associated with effort and with no aggravating or alleviating factors. He reported no respiratory or gastrointestinal problems, fever or history of trauma.
Physical examination on admission to the ED revealed no significant alterations. Diagnostic exams included an ECG, which showed sinus rhythm and right bundle branch block (RBBB), but no ST-segment changes. Laboratory tests revealed hemoglobin 11.5 g/dl, sodium 134 mmol/l, potassium 4.6 mmol/l, chloride 100 mmol/l, urea 56 mg/dl, creatinine 1.46 mg/dl (estimated glomerular filtration rate 61 ml/min/1.73 m2 according to the Chronic Kidney Disease Epidemiology Collaboration equation), and increased myocardial necrosis markers (high-sensitivity troponin I 348.5 μg/l at first assessment and 392.2 μg/l at second assessment, a rise of 13%).
A diagnosis of non-ST elevation MI was made, a loading dose of dual antiplatelet therapy (aspirin and clopidogrel) was administered and the patient was admitted. He presented no further episodes of chest pain, myocardial necrosis markers fell from 409.9 μg/l (peak) to 78.2 μg/l and there were no new ECG alterations.
Coronary angiography on the fourth day of hospital stay showed two-vessel disease (90% stenosis of the mid segment of the right coronary artery [RCA], and 95% stenosis of the first marginal), and two drug-eluting stents were successfully implanted.
The patient remained hemodynamically stable under dual antiplatelet therapy (aspirin 100 mg/daily and clopidogrel 75 mg/daily) and high-dose statin therapy (atorvastatin 40 mg). Bisoprolol and lisinopril were also started during hospital stay. The patient also received the same antiretroviral therapy he had been receiving as an outpatient.
Laboratory tests during hospitalization showed poor metabolic control, with glycosylated hemoglobin 9.2% (7.0% three months previously) and undetectable HBV and HIV-2 viral loads. The patient's lipid profile at admission was total cholesterol 142 mg/dl, HDL cholesterol 18 mg/dl, LDL cholesterol 52 mg/dl (by direct measurement), and triglycerides 326 mg/dl. Testing for thrombophilia included measurement of protein S, protein C, homocysteine, factor V Leiden, antithrombin and von Willebrand factor, all of which were within the reference range; screening for anti-nuclear antibodies and lupus anticoagulant was also negative.
The patient was discharged on the 11th day of hospitalization, under the medication described above and maintaining the antiretroviral therapy he was receiving at admission. There were no further complications.
He returned to the ED four hours after discharge, due to chest pain radiating to his left arm.
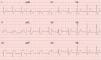
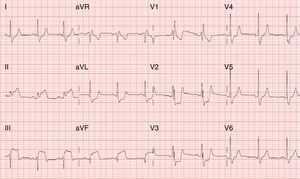
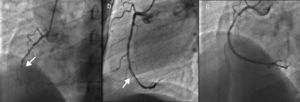
The ECG showed sinus rhythm, RBBB and ST elevation in leads DII, DIII, and aVF and ST depression in leads V1-V3 (Figure 1).
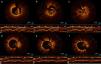
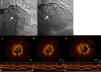
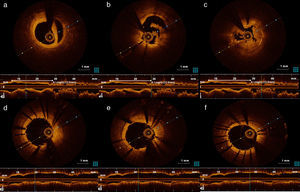
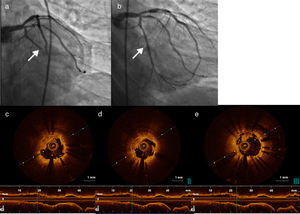
Coronary angiography was performed within an hour of pain onset, and revealed occlusion of both stents (Figure 2a and b). Optical coherence tomography (OCT) of the two arteries was performed to clarify the situation. OCT of the RCA showed good stent apposition, intrastent thrombus and dissection beginning at the distal margin of the stent (Figure 3a-c), while OCT of the first marginal showed good apposition and intrastent thrombi but no dissection (Figure 4a, c-e). Angioplasty of the mid segment of the RCA was then performed, and a stent was implanted after thrombus aspiration (Figure 3d-f), as well as balloon angioplasty of the first marginal after thrombus aspiration (Figure 4b). There was a good final result, TIMI 3 flow being re-established in both arteries. A glycoprotein IIb/IIIa inhibitor (abciximab) was administered, due to the patient's high thrombotic burden.
After the procedure it was decided to replace clopidogrel by ticagrelor. The patient experienced no recurrence of pain or new ECG alterations during the rest of hospital stay. He was discharged medicated with aspirin 100 mg once daily, ticagrelor 90 mg twice daily, bisoprolol 5 mg once daily and lisinopril 20 mg once daily.
At the time this case report was prepared, the patient had undergone cardiological assessment after four months of follow-up, and no further ischemic complications were recorded.
DiscussionThe increased life expectancy of HIV-infected patients following the introduction of HAART has been accompanied by an increased prevalence of metabolic disorders, which raises new problems for their management.
HIV and the pathophysiology of cardiovascular diseaseVarious factors are known to increase the risk of cardiovascular disease in HIV patients. On one hand, this population has a high prevalence of conventional risk factors,13 particularly smoking,13,14 low HDL cholesterol, hypertriglyceridemia and hypertension.13 Some studies15 report that the incidence of diabetes in HIV patients exposed to HAART is four times higher that in seronegative patients. On the other hand, complex pathophysiological mechanisms related to the virus itself also play a part, resulting from persistent immunodeficiency, immune dysregulation, immune activation and inflammation,16 even in patients under HAART.
Endothelial dysfunction, a key element of atherogenesis, is the end result of various processes that coexist in the presence of HIV, including direct and indirect endothelial injury (via immune reactions or drugs), destruction of CD4+ T cells (accompanied by an increase in shed membrane particles that induce endothelial dysfunction) and enhanced expression of adhesion molecules (via increased cytokine activity or as a direct effect of the virus).17,18
There is thus a persistent inflammatory state in these patients that results in vascular damage and premature atherosclerosis.19 Some studies have shown a significant increase in non-calcified plaques in HIV patients treated with HAART, which may also be associated with cardiovascular events.20
An increased risk of CAD has also been demonstrated in HIV patients compared to the general population, occurring at an earlier age3 and with more aggressive characteristics (greater prevalence of ST-elevation MI and multivessel involvement).2
In addition, HAART also causes metabolic changes that further increase risk in these patients, including metabolic syndrome, characterized by dyslipidemia (predominantly hypertriglyceridemia) and insulin resistance, frequently associated with abnormal fat distribution and lipodystrophy.21,22 This, while HAART helps to reduce endothelial injury by controlling HIV infection, it also activates the endothelium by interfering in glucose and lipid metabolism.23
Assessing the relationship between HAART and cardiovascular risk is complicated, since the currently recommended combined therapy regimes include drugs of various classes, making it difficult to draw reliable conclusions. In addition, many patients are medicated with different regimes over time, due to therapeutic failure or adverse effects.
Data from the D:A:D study,24 which analyzed the association between HAART and risk of MI, showed that of the protease inhibitors, only indinavir and lopinavir-ritonavir were associated with a significantly increased risk of MI.
In the same cohort, of nucleoside reverse transcriptase inhibitors, only abacavir and didanosine were significantly associated with risk of MI. There have been many studies on abacavir in particular, but with conflicting results. Some report an association between current or recent exposure to abacavir and increased risk of MI,25 while others found no evidence of such an association.26,27 There is in fact no known biological mechanism to explain the association, although experimental studies have suggested various potential explanations, including endothelial dysfunction and the ability of abacavir to induce inflammation of the vascular wall by enhancing leukocyte attachment to endothelial cells, and possibly to increase platelet activation.28
Incidence of acute-phase reinfarction or restenosis in HIV patientsThe prognosis of HIV patients in the acute phase of ACS has been analyzed in only a small number of works. A nationwide study in the USA between 1997 and 200629 found a higher rate of in-hospital mortality in HIV patients admitted with ACS (with or without ST elevation), although there were differences in the treatment offered in the seropositive and seronegative groups.
Some authors argue that HIV patients undergoing PCI present higher incidences of reinfarction, restenosis and stent thrombosis as a result of their prothrombotic state,2,30,31 although few other studies have demonstrated such an association. Hsue et al.32 reported a higher restenosis rate after PCI in HIV-infected patients than in controls (52% vs. 14%, p=0.006), but other studies10,11 have reported similar rates of cardiovascular adverse events in patients with and without HIV infection undergoing PCI with drug-eluting stents in the context of ACS.
Given the higher prothrombotic risk of these patients,33,34 it is important to investigate whether there is in fact a relationship between HIV infection and risk of stent thrombosis. In addition, there have been no studies assessing the impact of a more aggressive approach to treating conventional risk factors in HIV patients with a view to reducing their cardiovascular risk. Further studies are needed to increase our knowledge in areas such as reducing immune activation, chronic inflammation and residual viremia, and the possible relationship between antiretroviral therapy and stent thrombosis.
Particular aspects of the case reportedIn the case presented, even though the patient had other cardiovascular risk factors, HIV infection appears to be the main factor involved in stent thrombosis so soon after hospital discharge in an individual with optimized secondary prevention therapy.
One possible explanation for stent thrombosis in the RCA would be dissection of the artery that had not been visualized on initial coronary angiography and that contributed to the thrombotic event, since OCT revealed good apposition, intrastent thrombus and dissection beginning at the distal margin of the stent. Nevertheless, this is highly unlikely, since it does not explain thrombosis of two stents in different arteries.
The factor that could explain acute thrombosis of both stents is therefore increased thrombotic risk conferred by HIV infection and by diabetes.
There are no specific guidelines with regard to antiplatelet therapy in HIV-infected patients and the decision to replace clopidogrel by ticagrelor in this case was based not on evidence but on the failure of previous therapy. It would be interesting to know whether the new P2Y12 inhibitors are more effective at secondary prevention of cardiovascular events in HIV patients, and should therefore be used as first-line therapy.
ConclusionsThe transformation of HIV infection from a disease with high short-term mortality to a chronic disease has raised new and pressing research questions regarding the cardiovascular risk of this population, given their known risk profile.
The current approach to reducing cardiovascular risk consists of early initiation of HAART and adequate control of conventional risk factors.
However, the case presented highlights the fact that adequate control of HIV infection and conventional risk factors may be insufficient, and raises various questions that require thorough investigation.
Finally, a multidisciplinary approach will help in choosing the most appropriate care for these patients, with emphasis on the role of the cardiologist, infectologist and internist.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Carvalho AS, Osório Valente R, Almeida Morais L, Modas Daniel P, Sá Carvalho R, Ferreira L, et al. VIH e doença coronária – quando a prevenção secundária é insuficiente. Rev Port Cardiol. 2017;36:569.