The prevalence of heart failure has increased over the past decades and is a major social and economic burden on healthcare services. Patient quality of life is severely impaired and heart failure is one of the main causes of death in Portugal. The functional organization of multidisciplinary teams engaged in the treatment of these patients is essential to improve health care provision and outcomes, specifically reducing mortality, hospital admissions, and improving quality of life.
We describe current approaches to heart failure management and discuss the organization of heart failure units and cooperation among these units and also with other healthcare professionals.
A prevalência da insuficiência cardíaca tem vindo a aumentar nas últimas décadas, causando uma importante sobrecarga nos serviços de saúde, mas também económica. A qualidade de vida dos doentes fica muito comprometida e é uma das principais causas de morte em Portugal. A organização funcional das equipas multidisciplinares que seguem estes doentes é fundamental para a otimização dos cuidados prestados e melhoria dos resultados, em particular com redução da mortalidade, redução dos internamento e melhoria da qualidade de vida. Descrevem-se em seguida a abordagem contemporânea da insuficiência cardíaca, bem como uma discussão sobre a organização e articulação das unidades de insuficiência cardíaca entre si e com outras valências.
Heart failure (HF) is a highly prevalent disease with significant morbidity and mortality worldwide. Portugal is no exception.1,2 According to data from the Portuguese Health Directorate, HF was the second major source of hospital activity, accounting for 19000 admissions, lasting on average 10 days, and with high in-hospital mortality.2 Hospital admissions due to HF consume a high level of resources and readmissions at 30 days are also particularly significant.3–5 HF is a major economic and social burden in Portugal and also a serious public health problem.6 Since HF results from a chain of events that begins with cardiovascular risk factors, which have been on the rise, the burden of the disease is expected to increase progressively, exacerbated by the aging population, in whom the disease is more prevalent. In developed countries estimated prevalence of HF is around 2% of the general adult population.1 The Prevalence of Chronic Heart Failure in Southwestern Europe: the EPICA study, conducted in Portugal in 2002, estimated prevalence of HF in the adult population at 4.36%, rising to over 10% in the more advanced age ranges. More recent data are, however, not available.7 Recent estimates based on expected demographic change in Portugal predict an increase of around 30% in 2035 and 33% in 2060, compared to 2011. This corresponds to more than 450000 individuals with HF.8 The same estimates for hospital admissions suggest figures that will exceed 20000.8 Economic studies conducted in Portugal also point to an increase of 28% in the burden of HF between 2014 and 2036, representing 27059 Disability Adjusted Life Years and 8112 deaths, thus reinforcing the need to make HF a health priority in Portugal.9 Patients admitted for HF are at significant risk of readmission and mortality.3,4 Hospital admission is associated with a worse prognosis and accounts for approximately 70% of all the disease-associated costs.10
The development and setting up of clinics and units specializing in the assessment and treatment of HF are advocated in international recommendations on HF.11 They comprise acute HF in-patient units, which should include streamlined and systematic transition care leading up to patient discharge, agreements with primary health care providers, outpatient HF appointments, HF day hospitals and telemonitoring, including remote monitoring of outpatients. These professionals all require specific training. Studies have revealed the proven benefit of this multidisciplinary approach, such as good cost-effectiveness, and, in particular, improvements in patient quality of life, reductions in hospital admissions and readmissions, use of emergency departments, and mortality.12 Educating patients and caregivers is an essential part of this integrated process.
In contrast, easy access to several complementary diagnostic modalities, including advanced echocardiogram and cardiopulmonary stress testing, is essential in HF follow-up, as is access to cardiac rehabilitation, which according to national data is currently particularly weak.13 Wide-ranging access to more advanced therapies, including support from arrhythmology (cardioverter-defibrillators and cardiac resynchronization), coronary and structural interventional cardiology, coronary or valve heart surgery and heart transplantation or long-term ventricular assist devices is also important for these patients. The treatment of acute HF requires teams of experienced professionals to perform advanced and complex therapies. The main targets of these programs should be symptomatic and high risk patients. The programs should offer an optimized approach (both pharmacologically and the type of devices used); the patient and caregiver should be involved in the individual care and monitoring, and there should be regular follow-up (face-to-face appointments, telephone contact or telemonitoring), with easy access in the event of episodes of decompensated HF, and access to advanced treatment. The need for good support and access to palliative and end-of-life care is discussed much less.
The purpose of this review is two-fold. On the one hand, we aim to describe the current diagnosis and treatment of HF, particularly for those less familiar with this topic, and on the other, to raise awareness of and discuss the importance of a care referral network and proper liaison between stakeholders, the extension of the existing network and the need for an integrated approach, in line with international quality standards. Our intention is not to perform a systematic review of the literature or metanalysis, but to analyze the internationally recommended approach, based on consensus documents that were produced following an extensive review of references.
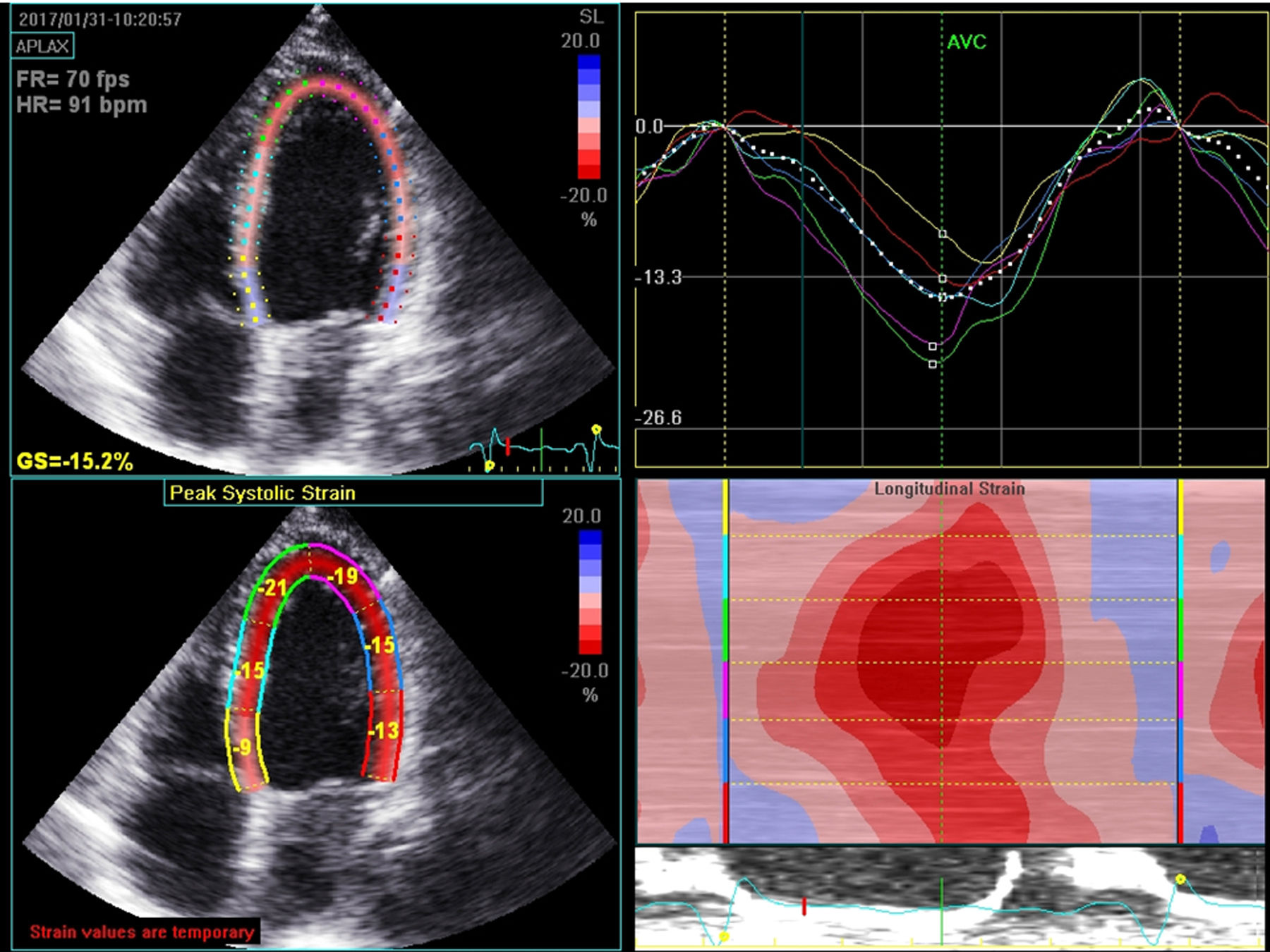
Diagnostic methodsEasy access to imaging techniques is essential to the diagnosis, etiological study, prognosis, choice of treatment and follow-up of patients with HF. Of the available methods, echocardiography is the preferred diagnostic tool, due to its low cost, wide availability, portability, and also due to the extensive information that can be obtained. Echocardiograms are therefore essential to the diagnosis of systolic or diastolic dysfunction, major valve disease, myocardial disease and right ventricular involvement.11,14 In addition to the traditional transthoracic echocardiography, new technology improves diagnostic capacity. For example, myocardial strain imaging using speckle tracking technology facilitates early detection of ventricular dysfunction in a subclinical phase; and three-dimensional echocardiography enables a more reliable assessment of left and right ventricular function with results that are very similar to those achieved through CMR imaging (Figure 1). The use of stress echocardiography allows the detection of myocardial ischemia, viability, contractile reserve and, in some cases, a more detailed assessment of valve disease. Although clinical trials have not shown any benefit to dyssynchrony evaluation in decisions on the use of cardiac resynchronization therapy (CRT), comprehensive echocardiography prior to the use of this therapy still plays an important role in this decision and also in the optimization of resynchronizer parameters. Recent studies have validated the role of stress echocardiography in assessing potential response to CRT. Parameters such as chronotropic response to stress with dobutamine, the presence of contractile reserve and the association between viability and contractile reserve appear to be promising parameters linked to a favorable response to resynchronization treatment.15–17
Cardiac magnetic resonance (CRM) imaging is still the gold standard for assessing left ventricular function, but due to its high cost, low accessibility and availability, it has been used more selectively, especially when echocardiography is inconclusive.11,14 It is particularly useful for assessing viability and identifying more uncommon HF etiologies due to late enhancement imaging.
Nuclear medicine can also be useful in specific situations, such as the diagnosis of ischemia and viability, and for example in the diagnosis of cardiac amyloidosis.11 Cardiac computerized tomography enables the assessment of coronary anatomy in patients with a low to intermediate pre-test probability of coronary artery disease.11,14
The use of more invasive methods, including coronary angiography, may be necessary when the diagnosis of myocardial ischemia is inconclusive using non-invasive testing or when the pre-test probability is high.11 In more advanced stages of the disease, full hemodynamic profiling is equally important to investigate the conditions and possible contraindications for more advanced treatment, such as heart transplantation.11,18
Selecting advanced chronic HF patients who may benefit from advanced therapy, such as heart transplantation and the placement of ventricular assist devices, requires a holistic assessment of patients. Although it is not the sole factor in the decision to refer patients for treatment with these therapies, the cardiopulmonary stress test is crucial and assesses the physiology and function of a series of organ systems. Many scientific associations, including the American Heart Association/American College of Cardiology and International Society for Heart and Lung Transplantation, recommend peak oxygen consumption in cardiopulmonary stress testing below 12 ml/kg/min as one of the main referral criteria for heart transplantation or placement of left ventricular assist devices.11,18 Other parameters, such as the VE/VCO2 slope and percentage of predicted peak oxygen uptake may be important for decisions in patients who cannot achieve maximal effort and in men and women under 50 years of age, respectively.11,18 These parameters may be better when determining risk and possibly more suited to listing patients for transplant than others. Table 1 illustrates the criteria for poor prognosis based on the cardiopulmonary stress test.
Poor prognosis criteria on the cadioplumonary stress test18
| Maximal cardiopulmonary exercise test (RER>1.05 and achievement of an anerobic threshold) |
| Peak VO2≤14 ml/Kg/min if not taking beta-blockersPeak VO2≤12 ml/Kg/min if taking beta-blockersYoung patients (<50 years) and women, include predicted % of peak VO2≤50% |
| In event of a submaximal cardioplumonary (RER≤1.05 or failure to achieve an anerobic threshold) |
| Use of ventilation equivalent of CO2 (VE/VCO2) slope of >35 |
CO2: carbon dioxide; Peak VO2: peak oxygen consumption; RER: Respiratory Exchange Ratio
As HF is an area that is constantly under clinical investigation, treatment options have evolved exponentially over the past 30 years. The available therapeutic arsenal, the use of which has been proven in clinical trials, includes pharmacological and non-pharmacological treatments. HF clinics should follow this evolution and offer the most up-to-date treatment possible to these patients at high risk of cardiac events.
Specialized teams with specific HF training are essential due to the poor prognosis of HF.19 Setting up these teams means treatment can be optimized and the benefits have been observed in several studies and systematic reviews of literature. These studies have shown an optimization of up to five times compared to treatment at the initial introduction of these units for the recommended therapies and a major reduction in HF admissions, which in some series reached almost 50%, although overall it is closer to 25%.3,20–24 There is very little data on experiences in Portugal.24 Data from neighboring Spain show that in 2011 only 37% of hospitals had HF units. Most of these were in level 3 hospitals (with hemodynamic monitoring, electrophysiology and cardiac surgery).25 The majority were managed by the cardiology department (84%) and 78% were general and non-advanced.25 These units also varied significantly in their programs, organization and activities and also in the tasks assigned to nursing staff.25 In 45% of the units, there were cardiac rehabilitation programs, 20% had a telemedicine program (telephone contact, telemonitoring of implantable devices or monitoring of biometric data with remote devices) and 64% had a day hospital. The setting analyzed in 2011 had changed very little since 2006, indicating that there was room for improvement.25 Based on the evidence and recommended standard of care for these patients, especially those admitted recently for decompensated HF, these specific multidisciplinary care programs should be implemented extensively.
Pharmacological therapyStudies have shown several drug groups can be used successfully in HF patients with reduced ejection fraction (EF). The publication of the CONSENSUS study in 1987 marked the start of the increase in available drug treatments. The benefits of angiotensin-converting enzyme inhibitors in lowering patient mortality were illustrated for the first time.26 Later, in 1999, two new pharmacological groups, beta-blockers (CIBIS-II trial) and mineralocorticoid-receptor antagonists (MRAs) (RALES trial) were also shown to reduce the mortality of these patients.27,28 In 2014 and 2019, following a 15-year hiatus, two new pharmacological groups evidenced a further reduction in mortality: sacubitril-valsartan in the PARADIGM-HF trial and SGTL-2 inhibitors in the DAPA-HF trial.29,30
Consequently, these four drug groups received a class I recommendation for their use in HF patients with reduced EF. The DAPA-HF trial with SGTL-2 inhibitors took place after the publication of the most recent recommendations from the European Society of Cardiology.11 Unfortunately, up until the present moment, the use of these pharmacological groups in HF patients with preserved EF has produced neutral results.
Other pharmacological groups are recommended in specific subpopulations given their association with reduced HF admissions (diuretics in patients with HF and congestive symptoms) or have been shown to reduce mortality (ivabradine in patients with sinus rhythm and a heart rate over 70 bpm, and isosorbide dinitrate/hydralazine in black individuals).31,32
Short-term treatment with inotropic agents in an acute phase of the disease improves hemodynamics and helps recovery from end-organ dysfunction.33 However, there are no proven benefits in terms of prognosis and they are therefore not indicated in the routine treatment of these patients. Their use is more limited as a bridge to other more advanced therapies.33 Inotropes are recommended in patients with low cardiac output, evidence of end-organ dysfunction and during decongestion. Vasopressors do not also have any benefit on prognosis and should be reserved for patients with low blood pressure and evidence of organ hypoperfusion.33 In the management of congestion, usually worsened by cardiorenal syndrome and diuretic resistance, loop diuretics should be administered, preferably intravenously, and if necessary in combination with other diuretics such as MRAs and metolazone.33 Ultrafiltration and peritoneal dialysis might be alternatives, the advantage of the latter being that it can be administered in an outpatient setting.33 Treating congestion essentially improves quality of life and reduces time spent in a hospital, but does not improve prognosis.33
Non-pharmacological therapyAn analysis of beneficial techniques for HF reveals that the treatment of these patients cannot be performed solely by an HF specialist. Support from various areas within cardiology and cardiac surgery is required.
Ischemic HF patients who are candidates for revascularization or those with HF caused by valve disease treatable with surgery require treatment defined in direct collaboration with the heart surgery team. Data from the Surgical Treatment for Ischemic Heart Failure (STICH) trial and its subtrials show that in the presence of multivessel disease, coronary artery bypass graft (CABG) or percutaneous coronary intervention (where complete revascularization is possible) significantly reduce mortality compared with drug therapy.34,35 Among patients with left ventricular dysfunction, CABG can be performed with acceptable 30-day mortality, thus maintaining the long-term benefit.34,35 Prior assessment of viability is essential when selecting patients who may benefit from this strategy.36 Revascularization of viable regions is associated with an improved prognosis. However, in multivariable analysis, this benefit is not sustained. Among experts, the consensus is that viability testing is still essential.
Advances in the devices available to arrhythmology teams have resulted in an arsenal of treatment possibilities for HF patients, including implantation of an implantable cardioverter-defibrillator (ICD), CRT, cardiac modulation therapies, atrial fibrillation ablation and ventricular extrasystole ablation associated with LV dysfunction.11
Interventional cardiology, which already played a fundamental role in the treatment of ischemic HF patients who are possible candidates for percutaneous revascularization, has illustrated through the treatment of functional mitral regurgitation that, in addition to its active role in the implantation of invasive monitoring devices, it has beneficial effects on patient mortality.11,37
Cardiac rehabilitation teams have signaled that not only is exercise safe for these patients but that it improves their quality of life, functional capacity and reduces hospital admissions.11 These multidisciplinary teams, comprised also of nurses, psychologists, dieticians and social workers, mean patient education is delivered in a more intensive fashion. Patients are directly involved in the treatment programs and have improved access to care during episodes of decompensation.11 In recent years, there has been an increase in centers with access to cardiac rehabilitation. This service, however, is still underused. Data from 2013-2014 show that only 8% of individuals with myocardial infarction were referred for phase two cardiac rehabilitation. Of all the patients referred for rehabilitation, HF was the main reason for referral in only 12.7% of cases. These figures were, however, an improvement on previous records.13
Heart failure day hospitalsDespite the recent development in ambulatory intravenous (IV) treatments for HF, there was already some evidence of their feasibility, benefit, safety and cost-benefit ratio. In a trial that included more than 100 subjects with decompensated heart failure, IV administration of diuretics in an ambulatory setting was safe, cost-effective and reduced hospital admissions.38 Another trial also demonstrated that ambulatory IV administration of loop diuretics according to standardized protocols relieved congestion effectively in decompensated HF patients across the whole EF spectrum.39
Other IV therapies given on an outpatient basis have been studied for improving HF symptoms and preventing hospital admissions. Pulsed infusions of levosimendan in outpatients with advanced HF were analyzed in two randomized clinical trials. In the LevoRep trial, levosimendan (0.2 μg/kg/min) vs. placebo administered for six hours at two-week intervals over six weeks did not significantly affect functional capacity and disease-related quality of life after 24 weeks.40 However, this study provided some indications on the safety and feasibility of an ambulatory treatment strategy, including the possibility of improved event-free survival with levosimendan. In the LION-Heart trial, a six-hour IV infusion of levosimendan (0.2 g/kg/min) every two weeks for 12 weeks, was associated with improved plasma N-terminal pro-brain natriuretic peptide concentrations, quality of life and HF admissions when compared with placebo.41 The intermittent use of levosimendan led to a reduction in hospital admissions and improved quality of life, howeverm there was no clear benefit on mortality. It is therefore indicated for symptomatic relief or as palliative care, but larger studies are needed to define its role better.
In iron-deficient HF patients, a metanalysis of four randomized clinical trials indicated that compared to placebo, the administration of IV ferric carboxymaltose reduces recurrent cardiovascular hospital admissions and is another possible ambulatory IV treatment.42
As part of outpatient clinic treatment (Figure 2), patient education must be boosted, equipping them with the necessary tools for self-monitoring and emphasizing the importance of adhering to their drug regimen, a healthy diet and raising awareness of the factors that lead to decompensation.
Monitoring of patients with heart failureThe theoretical potential benefits of patient monitoring in terms of reducing HF admissions have slowly been translated into a reality in clinical practice, due to the exponential growth in technology transmitting data in real-time. HF units currently have various strategies at their disposal and these together with a timely response may improve outcomes for this population. Even though the results from different clinical trials have been disparate, telemonitoring, in which a team receives information and is able to provide timely responses, may reduce HF admissions and all-cause mortality, as shown in the recent Telemedical Interventional Management in Heart Failure II trial (TIM-HF2).43
Remote monitoring is also possible with implantable devices such as ICDs and CRTs, enabling faster recognition of arrhythmias, device-related issues and worsening of HF signs. It is currently recommended when these devices are implanted.44 Although the changes recorded by these devices provide valuable information, direct assessment of filling pressure may provide additional data. The CHAMPION trial demonstrated this using the CardioMEMS™ device, which measures pressure directly in the pulmonary artery and showed that its use led to a reduction in HF hospital admissions.45
Advanced treatmentVentricular assist devicesMechanical ventricular assist devices are life-saving therapeutic options in acute and chronic HF.11,46,47 In advanced chronic HF, they are an alternative for individuals who are not candidates for transplantation as destination therapy; in individuals awaiting heart transplantation, but at high risk for morbidity and mortality, as a bridge to transplant; and in some individuals who are temporarily unsuitable candidates for transplant, as a bridge to candidacy.11
Mechanical ventricular assist devices significantly reduce mortality and morbidity in chronic end-stage HF patients.46 However, they may be associated with specific issues such as device or systemic thromboembolism, multifactorial bleeding, device-related infections, system failure, right ventricular failure, among others.46,47 These devices require specific and regular monitoring by multidisciplinary HF teams.
There have been progressive improvements to the design of assistance systems, including the change from pulsatile flow to continuous flow devices.47 More recently, the HeartMate 3™ device introduced a magnetically levitated design with centrifugal flow pump, which leads to a lower rate of system dysfunction and the need for removal.48 It is anticipated that gradual improvements in mechanical ventricular assist systems and their greater availability will increasingly contribute to addressing the issue of the growing number of patients with chronic end-stage HF facing contraindications for transplant and the widely acknowledged shortage of heart transplantation donors.47–49
For cardiogenic shock in acute HF, short-term mechanical circulatory support (MCS) devices can be used.33 These devices are usually placed percutaneously and can be used for several weeks to enable cardiac recovery and recovery of the target organs, or as a bridge to decision for long-term MCS or heart transplantation. Their use should be guided by device characteristics and also local team experience. The devices currently available are the intra-aortic balloon pump, extracorporeal membrane oxygenation (ECMO), TandemHeart™ and Impella™.33 The circulatory support provided for each one of the devices is variable and all require experience to be implanted. The intra-aortic balloon pump is easier to implant, however, its contribution to cardiac output is the lowest of all of the abovementioned devices. Its use has therefore been limited to an acute ischemic heart disease setting for support in high risk angioplasty, despite the evidence being limited.33
Heart transplantationIt is over 50 years since the first human-to-human heart transplantation and it is currently an established therapeutic option in advanced HF patients with reduced left ventricular EF.35 Heart transplantation involves a multidisciplinary team, including heart surgeons, cardiologists, internal medicine specialists, physical medicine and rehabilitation physicians, specialized nursing teams, psychologists and nutritionists, among other fields.50
One of the greatest challenges facing the teams is selecting suitable candidates for transplantation. History of the baseline disease and transplantation-associated morbidity and mortality must all be taken into account.51 In an acute HF setting, candidates for transplantation are patients in refractory cardiogenic shock, patients dependent on inotropic drugs or assist devices for adequate perfusion.52 In chronic HF, candidates for transplantation are patients with significant functional impairment and indicators of poor prognosis, such as cardiopulmonary stress tests (as previously mentioned), despite all pharmacological, percutaneous and surgical therapy and devices having been exhausted.51,52 However, this parameter must be correctly interpreted, its limitations must be known and it must be adapted to the clinical setting.53,54 In difficult decision-making processes, risk stratification scores from the Seattle Heart Failure Model or the Heart Failure Survival Score can be useful tools.55,56
The study of heart transplantation candidates includes an extensive assessment to rule out contraindications or conditions that increase risk after transplant beyond what is considered reasonable, such as infections, malignancies, lesions of other target organs or increased pulmonary vascular resistance.51 It is equally important to perform social assessments, to assess the patient's motivation, compliance with treatment and follow-up.51 It is noteworthy that, due to improved transplantation outcomes, the list of contraindications has been modified and currently patients with increased risk are candidates for surgery, whereas previously they had been excluded from this treatment.18 These advances are reflected in the updated recommendations from the International Society for Heart and Lung Transplantation, which include accepting patients of more advanced age, with higher body mass index and a higher degree of kidney dysfunction.18 However, the shortage of donors is recognized as a worldwide problem and appears to be increasing. It is not currently addressed by the development of ventricular assist therapies as destination therapies, nor by using marginal donors.
Currently, median one-year post-transplant survival is around 80 to 90%, with the median survival greater than 11 years and associated with good quality of life.53,57 In the initial post-transplant phase, the main causes of death include primary graft failure, infections and rejection; in the long-term they include graft vascular disease and malignancies.50,57 Diabetes, dyslipidemia, hypertension and chronic kidney failure are common.50,57 Post-transplant follow-up is very specific and requires competencies that go beyond strictly cardiac care.
Not only has surgical technique improved, but immunosuppressive therapy, antimicrobial prophylaxis and monitoring methods have been perfected.49 Immunosuppressive regimens vary considerably among sites.49 Maintenance immunosuppression usually includes the use of a calcineurin inhibitor (tacrolimus or cyclosporine), an antiproliferative agent (principally mycophenolate mofetil) and corticosteroids.50,58 mTOR inhibitors (everolimus, sirolimus), introduced more recently, have shown benefits in reducing cancer, kidney failure and graft vascular disease.50,58 The overall objective is to use an immunosuppressive regimen at the lowest dosage possible to avoid its adverse effects, such as infections, malignancies, nephrotoxicity and metabolic dysregulation, but enough to prevent acute cellular rejection.49,50,58 Regimens with corticosteroid suppression are used in some cases; tacrolimus monotherapy has been tested and there have been promising results.49,50,58,59
The standard of care for detecting rejection is still an endomyocardial biopsy normally in the first year after the transplantation.50 In addition to acute cell rejection, humoral rejection is currently an established entity requiring specific studies.49,50 Extensive research has been conducted into the use of non-invasive methods for detecting rejection. These include imaging techniques, different echocardiography methods and magnetic resonance and biomarkers, including gene expression profiling in circulating mononuclear cells.49,60,61 The results are promising, but the diagnostic precision of each of these methods is not a substitute for endomyocardial biopsies.49,60,61
Due to the risk of graft vascular disease, one of the main long-term causes of death, regular surveillance is required using invasive coronary angiography and ideally imaging techniques, such as invasive coronary angiography, given the specific manifestation of this type of coronary disease.49,50 Non-invasive methods have been studied, but until the present moment they are not a substitute for regular invasive surveillance.49,50
In summary, multidisciplinary teams are required in heart transplantation, especially for candidate selection and in the complex post-transplantation follow-up. Skills are required beyond those strictly limited to cardiac care, which has its own specific characteristics.
Unresolved issuesAs a rule, palliative care has rarely been addressed or discussed. The advent of new forms of treatment has meant there are more treatment options available, resulting in improved patient outcomes and quality of life. However, there are still cases with unfavorable progression, in which easy access to palliative care is vital, thus making cooperation with these units essential. Most patients reject or do not acknowledge the terminal nature of HF, focusing primarily on a day-to-day approach, despite clear clinical worsening. End-of-life approaches should be discussed with a multidisciplinary team, including palliative care specialists and psychologists so that the patient has a well-established and defined end-of-life.62
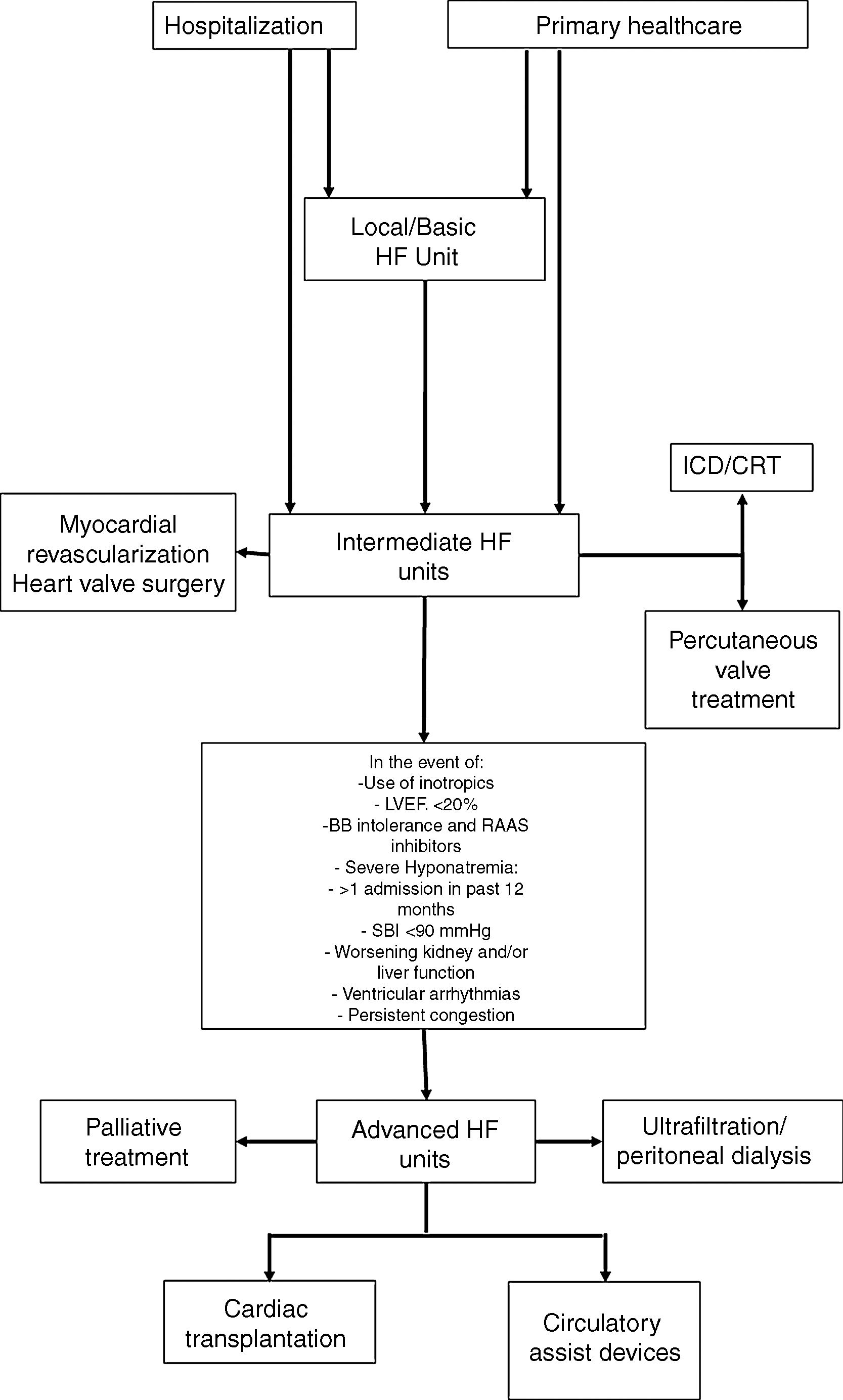
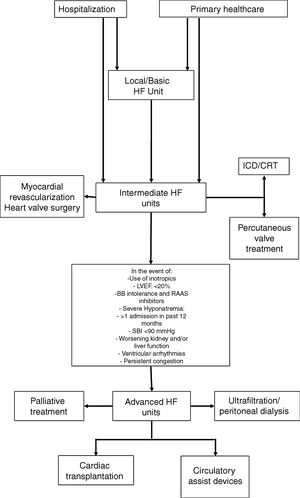
In view of the different patterns of clinical progression and very variable care needs, these patients will require different levels of care throughout the course of their disease, and their needs will be diverse, both in terms of medical support and nursing, but also the technology used to care for them. These issues have already been debated for other acute or critical diseases, such as trauma, and also for intensive cardiac care. For acute diseases, the classification of the patients according to three levels of severity is recommended; intensive care units are also classified as level I (basic), II (intermediate) and III (advanced), based on the expertise of the staff and techniques available at each unit.63 This approach enables us to obtain more information on how to care for these patients, allocate resources more efficiently and improve outcomes. In an HF treatment setting, profiling the centers based on available human and technical resources, together with the definition of the care required by the patient, may allow them to be included in a referral network for HF treatment in tertiary hospitals and other hospitals, resulting in more efficient use of resources (Figure 3) and a clear definition of when to refer patients to an advanced HF treatment unit.33
HF guidance algorithm for treating HF patients.
Legend: BB: beta-blockers; CRT: cardiac resynchronization therapy: HF: heart failure; ICD: implantable cardioverter-defibrillator; LVEF: left ventricle ejection fraction; RAAS: renin-angiotensin-aldosterone system; SBP: systolic blood pressure.
Referral for specified treatment to intermediate HF units may also be made from Basic HF Units (via agreements with other hospitals) or by the advanced HF units.
To achieve greater efficiency when treating HF patients, the existence of guidelines is particularly important (especially “an integrated care process for HF management”) as is the definition of quality indicators for assessing performance relating to the provision of medical care to HF patients and for constructive improvement of care, as has happened in other countries, such as Spain.64 A clearly defined approach in line with internationally recommended standards is essential in order to standardize clinical practice in Portugal. The definition of a national referral network for the treatment of chronic and acute HF might enable the faster provision of appropriate guidance for these patients. Finally, conducting internal and external audits allows us to identify areas that need to be improved locally or nationally, thus permitting a more appropriate use of resources that may be very scarce in some places.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Timóteo AT, Pereira Silva T, Ilhão Moreira R, Gonçalves A, Soares R, Cruz Ferreira R. Unidades de insuficiência cardíaca: estado da arte na abordagem da insuficiência cardíaca. Rev Port Cardiol. 2020;39:341–350.