The association between heart disease and pregnancy is increasingly prevalent. Although most women with heart disease tolerate the physiological changes of pregnancy, there are heart conditions that manifest for the first time during pregnancy and others that totally contraindicate a pregnancy. It is therefore important to establish multidisciplinary teams dedicated to the management of women with heart disease who intend to become, or who already are, pregnant. The aim of this article is to systematically review current knowledge on the approach to women with high-risk cardiovascular disease during pregnancy.
A associação entre cardiopatia e gravidez é cada vez mais frequente. Ainda que a grande maioria das mulheres com doenças cardíacas tolere as alterações fisiológicas da gravidez, existem patologias cardíacas que se manifestam pela primeira vez durante o estado gravídico e outras que contraindicam totalmente uma gravidez pelo risco materno que lhe está associado. Desta forma, torna-se premente a criação de equipas multidisciplinares dedicadas à abordagem de mulheres com doença cardíaca que pretendem engravidar ou que já estão grávidas. O objetivo deste artigo é sistematizar, com base no conhecimento atual, a abordagem de mulheres com doença cardiovascular de alto risco durante a gravidez.
The spectrum and prevalence of heart disease in pregnancy varies considerably between countries. According to the most recent information, 1-4% of all pregnancies in industrialized countries are complicated by cardiovascular disease (CVD).1 This incidence has increased, due to women becoming pregnant at older ages, the higher prevalence of cardiovascular risk factors (smoking, diabetes, obesity and hypertension) in women of child-bearing age, and the growing number of women with corrected congenital heart disease (CHD) who reach adulthood. In a study which included 13 Canadian cardiology centers, CHD accounted for 80% of all heart disease in pregnancy,2 while in a registry at the Heart Institute of the University of São Paulo, Brazil, that included 1000 pregnant women with heart disease followed for 10 years, the most common etiology was rheumatic heart disease, found in 55% of cases.3
Cardiomyopathies are rare in pregnancy, although they are an important cause of complications, peripartum cardiomyopathy (PPCM) being responsible for the most serious adverse events.4
Studies suggest that pregnancy-related mortality has increased in recent decades, with a growing number of deaths attributable to CVD,5 which is currently the leading cause of maternal death in Western countries.6
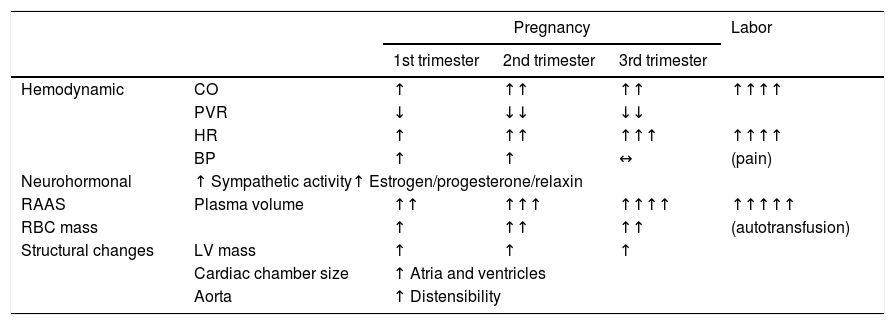
Hemodynamic adaptations to pregnancyImportant adaptations to the cardiovascular system occur in response to pregnancy to meet the increasing metabolic needs of both mother and fetus (Table 1). Non-adaptive hemodynamic changes can lead to maternal and fetal morbidity.7 Failure of normal adaptations can lead to decompensation of existing heart disease, the onset of symptoms, or the first manifestations of previously unknown disease, and hence pregnancy is often considered a natural stress test.
Physiological changes in pregnancy (adapted from Sanghavi et al.7).
| Pregnancy | Labor | ||||
|---|---|---|---|---|---|
| 1st trimester | 2nd trimester | 3rd trimester | |||
| Hemodynamic | CO | ↑ | ↑↑ | ↑↑ | ↑↑↑↑ |
| PVR | ↓ | ↓↓ | ↓↓ | ||
| HR | ↑ | ↑↑ | ↑↑↑ | ↑↑↑↑ | |
| BP | ↑ | ↑ | ↔ | (pain) | |
| Neurohormonal | ↑ Sympathetic activity↑ Estrogen/progesterone/relaxin | ||||
| RAAS | Plasma volume | ↑↑ | ↑↑↑ | ↑↑↑↑ | ↑↑↑↑↑ |
| RBC mass | ↑ | ↑↑ | ↑↑ | (autotransfusion) | |
| Structural changes | LV mass | ↑ | ↑ | ↑ | |
| Cardiac chamber size | ↑ Atria and ventricles | ||||
| Aorta | ↑ Distensibility | ||||
↑: increased; ↓: decreased; ↔: no change; BP: blood pressure; CO: cardiac output; HR: heart rate; LV: left ventricular; PVR: peripheral vascular resistance; RAAS: renin-angiotensin-aldosterone system; RBC; red blood cell.
Blood volume increases considerably during pregnancy, rapidly between six and 20 weeks, and less markedly between 20 weeks and term, with a mean overall increase of around 50%.8 Increased erythropoietin production stimulates erythropoiesis, which can rise by over 40% in a pregnant woman without nutritional deficiencies.9 However, the increase in plasma volume is greater than that of red blood cell mass, resulting in hemodilution, which leads to physiological anemia of pregnancy. Hemoglobin levels as low as 11 g/dl are considered physiological.7 Cardiac output (CO) rises by around 50%, initially due mainly to increased systolic volume and then to increased heart rate (HR) in the third trimester. Peripheral vascular resistance (PVR) falls during pregnancy, leading to reductions in systolic and diastolic blood pressure (BP). BP is lowest during the second trimester (5-10 mmHg below initial levels), although the steepest BP falls occur between six and eight weeks of pregnancy.10 As pregnancy-related changes in BP occur very early, BP levels later in the pregnancy should be compared with those before pregnancy, rather than with those recorded in the first weeks.7 During the third trimester, BP returns to pre-conception levels.
CO reaches a peak in labor and immediately after delivery, with an increase of 60-80%. This is due to various factors, particularly higher HR, increased preload associated with uterine contractions (for each uterine contraction 300-500 ml of blood enters the systemic circulation), and elevation of circulating catecholamines.11 It is extremely important to maintain blood volume at this stage and care should be taken to avoid excessive blood loss, which could drastically reduce preload. This is the stage at which there is greatest risk of decompensation of heart disease.
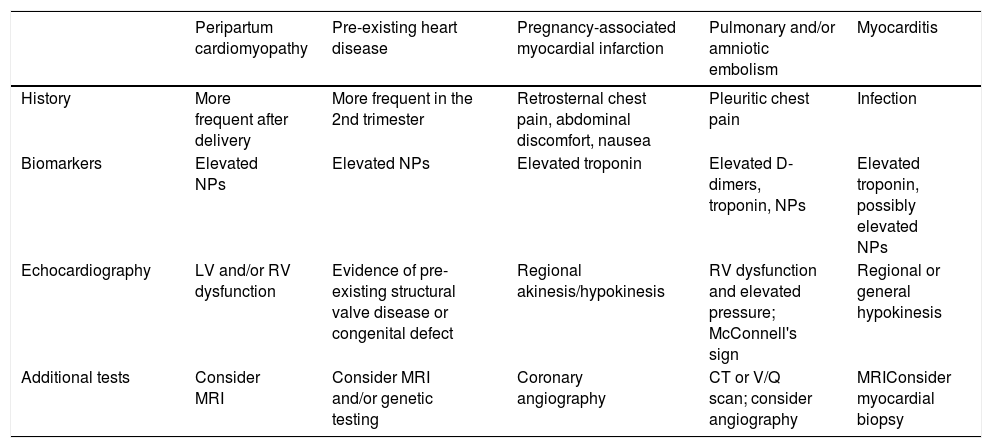
Diagnosis of cardiovascular disease in pregnancyA complete medical history is essential, focusing on characterization of the symptoms and signs associated with the physiological changes of pregnancy. Healthy pregnant women may present exertional dyspnea, fatigue and palpitations. On physical examination, lower limb edema and jugular venous distension are common findings. On cardiac auscultation, after the first trimester the first sound is louder, and a ejection systolic flow murmur, a third sound and an atrioventricular diastolic flow murmur are heard in 90%, 80% and 20% of cases, respectively.7 However, chest pain, new-onset dyspnea, symptomatic hypotension, unexplained tachycardia, palpitations associated with syncope, and cyanosis should be always considered warning signs. Differential diagnosis should be based on a detailed clinical history, with additional examinations being ordered according to clinical suspicion, weighing their risk and benefit and interpreting the results in the clinical context, as shown in Table 2.12
| Peripartum cardiomyopathy | Pre-existing heart disease | Pregnancy-associated myocardial infarction | Pulmonary and/or amniotic embolism | Myocarditis | |
|---|---|---|---|---|---|
| History | More frequent after delivery | More frequent in the 2nd trimester | Retrosternal chest pain, abdominal discomfort, nausea | Pleuritic chest pain | Infection |
| Biomarkers | Elevated NPs | Elevated NPs | Elevated troponin | Elevated D-dimers, troponin, NPs | Elevated troponin, possibly elevated NPs |
| Echocardiography | LV and/or RV dysfunction | Evidence of pre-existing structural valve disease or congenital defect | Regional akinesis/hypokinesis | RV dysfunction and elevated pressure; McConnell's sign | Regional or general hypokinesis |
| Additional tests | Consider MRI | Consider MRI and/or genetic testing | Coronary angiography | CT or V/Q scan; consider angiography | MRIConsider myocardial biopsy |
CT: computed tomography; LV: left ventricular; MRI: magnetic resonance imaging; NPs: natriuretic peptides; RV: right ventricular; V/Q: ventilation/perfusion.
The negative predictive value of natriuretic peptides is maintained during pregnancy, and they have been shown to help exclude heart disease in pregnant women. However, changes in B-type natriuretic peptide levels in pregnancy, and their prognostic impact in pregnant women with heart disease, remain the subject of debate.13
The majority of pregnant women have a normal electrocardiogram (ECG), but elevation of the diaphragm by the pregnant uterus can lead to left axis deviation of 15-20°. Other possible non-pathological electrocardiographic findings are transient ST-segment and T-wave changes, presence of a Q wave and inverted T waves in DIII, an attenuated Q wave in aVF, and inverted T waves in V1, V2 and, occasionally, V3.14
Transthoracic echocardiography (TTE) is the gold standard for assessing cardiac function during pregnancy. Non-pathological findings in a pregnant woman include mild dilatation of all four chambers (which may be more marked in the right atrium and ventricle), transient trivial mitral regurgitation (MR), physiological pulmonary and tricuspid regurgitation (TR),7 and increases in CO and left and right ventricular mass.15 Aortic regurgitation (AR) is always pathological.16 Transesophageal echocardiography can be useful for the characterization of CHD and when aortic dissection or prosthetic valve dysfunction are suspected, particularly for diagnosing vegetations and thrombi.
If examinations involving ionizing radiation are called for, this decision requires careful consideration, because even though the priority is the mother's health, the effects on the fetus must also be taken into account. The radiation dose to which the fetus, which is protected by the uterus, is exposed tends to be lower than that received by the mother, although the fetus is more sensitive. The effects depend on the radiation dose and on gestational age; if possible the exam should be postponed until after the first 12 weeks of pregnancy, the period of major organogenesis. There is no evidence that doses of <50 mGy are associated with increased risk of miscarriage, congenital malformation, growth restriction or mental problems (https://emergency.cdc.gov/radiation/prenatalphysician.asp). The dose to which a fetus is exposed from a chest X-ray is <0.01 mGy, but even so, an X-ray should only be performed if no other examination can clarify the etiology of the mother's symptoms. Computed tomography is rarely used for diagnosis of CVD in pregnancy and, given the high radiation doses required, is not recommended. An exception can be made if the mother's survival is at stake (Table 2). Cardiac magnetic resonance imaging (MRI) appears to be safe for both mother and fetus17 and can be useful for characterizing complex heart disease and disease of the aorta. The risk to the fetus from exposure to gadolinium is not known and gadolinium contrast should therefore not be used.14
Stress testing, either exercise or pharmacological, should also be avoided in pregnancy due to the risk of hypoxemia, fetal bradycardia and even fetal loss, caused by reduced placental blood flow. Pre-conception stress testing plays an important role in assessing functional capacity, chronotropic and blood pressure response to exertion, and exertion-induced arrhythmias in the monitoring of patients with CHD and asymptomatic valve disease.14 Stress echocardiography can be useful for pre-conception assessment of myocardial contractile reserve in women with previous PPCM and recovery of left ventricular ejection fraction (LVEF), other cardiomyopathies with slightly impaired LVEF, valve disease, and CHD.
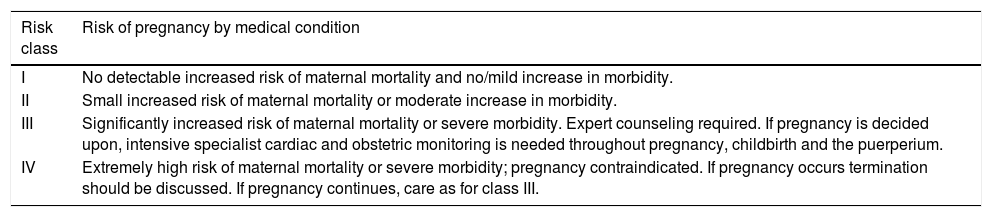
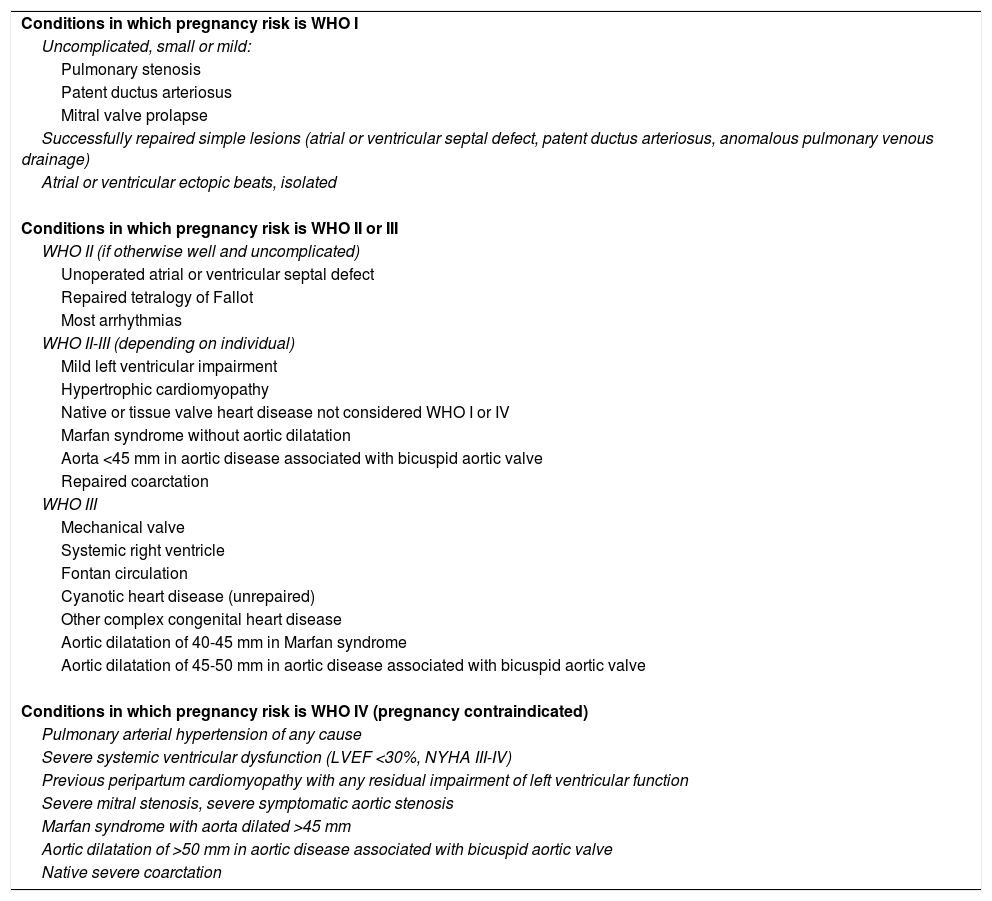
Risk stratification of pregnant women with cardiovascular diseaseAssessment of the risk of pregnancy in women with known CVD should be individualized and ideally performed before pregnancy, including adjustments to medication such as suspending contraindicated drugs and introducing alternatives. Several scores have been created to stratify the risk of cardiovascular complications in pregnancy, the most commonly used of which is the Cardiac Disease in Pregnancy (CARPREG) risk score.18 The European Society of Cardiology (ESC) guidelines recommend assessment of the risk of cardiovascular complications based on the classification of the World Health Organization (WHO), as this includes predictors that are not incorporated in the CARPREG and other risk scores (Tables 3 and 4).14
Modified World Health Organization classification of maternal cardiovascular risk: principles (adapted from Regitz-Zagrosek et al.14).
| Risk class | Risk of pregnancy by medical condition |
|---|---|
| I | No detectable increased risk of maternal mortality and no/mild increase in morbidity. |
| II | Small increased risk of maternal mortality or moderate increase in morbidity. |
| III | Significantly increased risk of maternal mortality or severe morbidity. Expert counseling required. If pregnancy is decided upon, intensive specialist cardiac and obstetric monitoring is needed throughout pregnancy, childbirth and the puerperium. |
| IV | Extremely high risk of maternal mortality or severe morbidity; pregnancy contraindicated. If pregnancy occurs termination should be discussed. If pregnancy continues, care as for class III. |
Modified World Health Organization classification of maternal cardiovascular risk: application (adapted from Regitz-Zagrosek et al.14).
| Conditions in which pregnancy risk is WHO I |
| Uncomplicated, small or mild: |
| Pulmonary stenosis |
| Patent ductus arteriosus |
| Mitral valve prolapse |
| Successfully repaired simple lesions (atrial or ventricular septal defect, patent ductus arteriosus, anomalous pulmonary venous drainage) |
| Atrial or ventricular ectopic beats, isolated |
| Conditions in which pregnancy risk is WHO II or III |
| WHO II (if otherwise well and uncomplicated) |
| Unoperated atrial or ventricular septal defect |
| Repaired tetralogy of Fallot |
| Most arrhythmias |
| WHO II-III (depending on individual) |
| Mild left ventricular impairment |
| Hypertrophic cardiomyopathy |
| Native or tissue valve heart disease not considered WHO I or IV |
| Marfan syndrome without aortic dilatation |
| Aorta <45 mm in aortic disease associated with bicuspid aortic valve |
| Repaired coarctation |
| WHO III |
| Mechanical valve |
| Systemic right ventricle |
| Fontan circulation |
| Cyanotic heart disease (unrepaired) |
| Other complex congenital heart disease |
| Aortic dilatation of 40-45 mm in Marfan syndrome |
| Aortic dilatation of 45-50 mm in aortic disease associated with bicuspid aortic valve |
| Conditions in which pregnancy risk is WHO IV (pregnancy contraindicated) |
| Pulmonary arterial hypertension of any cause |
| Severe systemic ventricular dysfunction (LVEF <30%, NYHA III-IV) |
| Previous peripartum cardiomyopathy with any residual impairment of left ventricular function |
| Severe mitral stenosis, severe symptomatic aortic stenosis |
| Marfan syndrome with aorta dilated >45 mm |
| Aortic dilatation of >50 mm in aortic disease associated with bicuspid aortic valve |
| Native severe coarctation |
LVEF: left ventricular ejection fraction; NYHA: New York Heart Association functional class; WHO: World Health Organization risk classification.
The type of delivery should be decided and scheduled by a multidisciplinary team. The preferred mode of delivery is vaginal, with a delivery plan individualized to the patient, her disease, and her hemodynamic profile. Cesarean section, although controversial, is indicated for patients with conditions in WHO risk group IV, under oral anticoagulation in pre-term labor, with decompensated heart failure (HF), or for obstetric indications.14 Tocolytic beta-agonists should not be used in mitral stenosis (MS) since by inducing tachycardia, they reduce left ventricular (LV) filling time and consequently increase left atrial pressure. Alternatively, atosiban, an oxytocin antagonist, can be used. Corticosteroids are contraindicated in patients with decompensated heart disease due to the risk of pulmonary congestion, pulmonary edema (PE) and cardiogenic shock.
Infective endocarditisThe ESC19 and the American College of Cardiology/American Heart Association (ACC/AHA)20 do not recommend antibiotic prophylaxis during vaginal or cesarean delivery. However, in the Brazilian Society of Cardiology guidelines prophylaxis against infective endocarditis is indicated in high-risk patients, with 2 g ampicillin associated with 1.5 mg/kg gentamicin one hour before birth. In allergic patients, penicillin should be replaced by 1 g vancomycin.21
Valvular heart diseaseStenotic and left-sided valve lesions are at higher risk of decompensation in pregnancy than regurgitant and right-sided lesions. Valve stenosis restricts increases in CO, raising transvalvular gradients and pressures upstream of the lesion, and is therefore less well tolerated in pregnancy than regurgitation, since regurgitant volume diminishes with systemic vasodilation and consequent reduced afterload. Mechanical heart valves are associated with specific problems (see below).
Although most women with less severe valve disease tolerate pregnancy well, some valve lesions are considered prohibitive: severe MS, severe symptomatic aortic stenosis (AS), and any valve disease associated with LV dysfunction (LVD) and/or pulmonary hypertension (PH). Women with these conditions should receive pre-conception counseling and should be treated before pregnancy. Hemodynamic changes in pregnancy can lead to increased mitral and aortic valve gradients on TTE, leading to overestimation of the severity of the valve lesion,22 and so stenosis should be quantified by valve area assessed using planimetry, or by pressure half-time for MS or by the continuity equation for AS.23,24 For women who remain stable during pregnancy, term delivery is recommended. Vaginal delivery with good pain management is the preferred method for most women with valve disease. Some experts suggest a cesarean section for patients with severe AS.14
Mitral stenosisMS is the most common valve disease in women of child-bearing age, and in 90% of cases is of rheumatic etiology. The hemodynamic changes associated with pregnancy (higher HR, CO, plasma volume and red blood cell mass) lead to increased left atrial pressure and PE. Many patients with MS become symptomatic for the first time during pregnancy. The most common complications are reduced functional capacity, arrhythmias (most often atrial fibrillation [AF]) and PE. These are related to mitral valve area and NYHA class24 and occur more often in the second and third trimesters, when hemodynamic changes are more marked.25 If symptoms occur, the patient should be started on beta-blockers, to prolong ventricular filling time and reduce left atrial pressure, and, if necessary, diuretics to relieve congestion. Anticoagulation is indicated in the presence of AF, atrial thrombi or a history of thromboembolism. Percutaneous mitral commissurotomy should be considered only when, despite medical treatment, the patient remains in NYHA class III/IV, and preferably not until after 20 weeks gestation. Cardiac surgery should be reserved for life-threatening situations in which all other measures have failed.14
Mitral regurgitationThe most common causes of MR in pregnant women are rheumatic valve disease, MVP and CHD. Reduced PVR and BP during pregnancy explain why women with mild, moderate or even severe MR but without LV dilatation or dysfunction tolerate pregnancy well. However, increased plasma volume and CO can lead to HF or arrhythmias, particularly in cases of severe MR and in patients with LV dilatation or dysfunction.22
Aortic stenosisBicuspid aortic valve is the main cause of AS in women of child-bearing age, and is frequently associated with aortic dilatation and coarctation, which further increases the risk in pregnancy. Mild to moderate AS is generally well tolerated, unlike severe AS, which is associated with angina, tachyarrhythmias and PE. Unlike MS, there is no effective pharmacological therapy for AS. Pulmonary congestion can be relieved with diuretics, although these should be avoided as much as possible due to the risk of hypotension and reduced placental blood flow. Where there are signs and symptoms of HF, syncope or angina, percutaneous or surgical intervention is indicated.23
Aortic regurgitationLike AS, the most common cause of AR in young women is bicuspid aortic valve. Women with severe AR and preserved systolic function usually tolerate pregnancy well. However, severe AR associated with LVD due to increased CO and plasma volume is poorly tolerated. Symptomatic pregnant women should receive HF therapy.22
Pulmonary stenosisIsolated pulmonary stenosis is more common when there are congenital abnormalities of the pulmonary valve. Even in women with severe pulmonary stenosis, cardiac complications (HF and low CO) during pregnancy are rare, but if present they can be treated by percutaneous valvuloplasty, with good results at any gestational age.26 Non-cardiac complications have been reported, including hypertensive disorders, prematurity and thromboembolic complications.27
Tricuspid regurgitationThe causes of primary, non-trivial, TR in young women include CHD (for example Ebstein's anomaly, which, depending on its complexity, can affect the prognosis), rheumatic valve disease, and infective endocarditis. TR is usually well tolerated during pregnancy. However, in surgically corrected or uncorrected CHD in which the tricuspid is the only atrioventricular valve, the valve becomes regurgitant and may be associated with ventricular dilatation and dysfunction, which increases the pregnancy risk.28
Prosthetic valvesWhen a woman who may become pregnant has a native valve replaced, the risks and benefits of a biological valve (risk of structural deterioration and less durability, with 90% likelihood of reintervention for valve replacement after 15 years,29 but with no need for anticoagulation) need to be weighed against those of a mechanical valve (greater durability and better hemodynamic profile, but higher risk of thromboembolism and consequent need for lifelong anticoagulation). Pregnancy is usually well tolerated in women with biological valves; maternal cardiovascular risk depends on valve and ventricular function to a similar extent to native valve disease. Monitoring of the pregnancy is similar to that for native valve disease. The risk of valve thrombosis, bleeding and fetal complications is higher with mechanical valves.
A retrospective study published in 2015 of 84 pregnant women with valve disease (23 of whom had prosthetic valves) demonstrated that pregnancy in women with prosthetic valves was associated with high maternal and fetal morbidity.30 2015 also saw publication of data on the outcomes of pregnancy in women with prosthetic valves from the ESC's Registry Of Pregnancy And Cardiac Disease (ROPAC).31 This registry included 212 patients with mechanical valves, 134 with biological valves and 2620 without a prosthetic valve. Maternal mortality was 1.5% in the group with prosthetic valves and 0.2% in women without (p=0.025). Valve thrombosis occurred in 10 women with mechanical valves, and bleeding complications were also more common in this group (23% vs. 5% both in women with biological valves and in those without a prosthetic valve, p<0.001). Event-free survival was 78% in women without prosthetic valves, 79% in those with biological valves and only 58% in those with mechanical valves (p=0.001). Furthermore, fetal outcomes of mothers with mechanical valves was worse, with significantly higher incidences of miscarriage, death and lower birthweight. Many specialists therefore prefer to replace the native valve with a biological valve in women who wish to become pregnant, not only because of the lower associated risk, but also because studies have demonstrated that pregnancy does not affect degeneration of biological valves,32 and because they permit percutaneous valve-in-valve treatment, which is likely to be required for intermediate- and high-risk patients in the future.
AnticoagulationPregnancy is a prothrombotic state, due not only to the venous stasis with which it is associated, but also to hypercoagulability resulting from increasing levels of thrombogenic factors throughout pregnancy.33 However, the maternal and fetal complications associated with different anticoagulation regimens, and the lack of randomized clinical trials and consistent guidelines, mean that management of anticoagulation during pregnancy is problematic.22
Warfarin, a vitamin K antagonist, crosses the placenta, and its use in the first 6-12 weeks of pregnancy is associated with fetal complications, including warfarin embryopathy (1-30%) and miscarriage (15-56%),22 and a higher incidence of miscarriage and fetal intracranial bleeding throughout pregnancy,30 the incidence of which varies in different studies.34,35 However, when maintained throughout pregnancy, it offers the best thromboembolic protection in women with mechanical valves. Although they included few patients, studies have shown that the risk of fetal toxicity is lower when therapeutic anticoagulation is achieved with warfarin doses ≤5 mg/day rather than with higher doses.36,37 On the basis of these findings, the ESC and ACC/AHA consider vitamin K antagonists safe, recommending warfarin <5 mg/day (or phenprocoumon <3 mg/day or acenocoumarol <2 mg/day) throughout pregnancy (ESC: class IIa recommendation, level of evidence C; AHA/ACC: class IIa, level B).14,38 When the dose needed to achieve the target INR is higher than those above, vitamin K antagonists should be replaced by continuous low molecular weight heparin (LMWH) or unfractionated heparin (UFH) during the first trimester, the critical stage of organogenesis. However, this approach is debatable, because other studies have demonstrated that warfarin is associated with fetal mortality even at low doses.39
The AHA/ACC recommend aspirin 75-100 mg/day in association with warfarin during the second and third trimesters.38
UFH does not cross the placenta and thus has no direct effect on the fetus. Subcutaneous administration is not effective, and is not therefore recommended due to the risk of thromboembolic complications,40 but intravenous UFH is the most appropriate form of anticoagulation pre- and post-birth due to its rapid onset of action and elimination.
Like UFH, LMWH does not cross the placenta, but has a better safety profile (greater bioavailability and longer half-life, and lower risk of bleeding and of heparin-induced thrombocytopenia).40 However, its efficacy during pregnancy is debatable. Some studies have shown similar efficacy to that of warfarin in women with mechanical valves if administered at the correct dosage (twice daily based on the mother's body weight) and with anti-Xa levels measured 4-6 hours after administration, for a target value of 0.8-1.2 U/ml.35 However, a study of 15 pregnant women receiving full-dose LMWH (1 mg/kg±20% subcutaneously twice daily) showed that factor anti-Xa levels were subtherapeutic in over 50% of cases and that levels varied considerably between administrations.41
Warfarin should be suspended at least a week before delivery, in a hospital environment, and should be switched to LMWH or UFH. If warfarin is replaced by LMWH, the latter should be suspended 36 hours before delivery and UFH started. In turn, UFH should only be suspended 4-6 hours before the birth and resumed 6-8 hours afterwards if hemostasis is ensured. The management of warfarin therapy varies between centers but it should be resumed 48 hours after childbirth.
Use of the new oral anticoagulants is increasing in non-pregnant women. The US Food and Drug Administration recently gave rivaroxaban a class C recommendation in pregnancy, but there have as yet been no reports on its use.42
Complex congenital heart diseaseCHD accounts for 80% of heart disease in pregnant women in the Western world.43 Some categories of CHD, such as Fontan circulation, systemic right ventricle and uncorrected cyanotic CHD, are associated with high maternal and fetal risk. In patients with Fontan circulation, 10% of pregnancies are associated with maternal complications, the most common of which are arrhythmias, and there may also be thromboembolic complications and worsening of HF.44,45 There is agreement that Fontan patients with impaired ventricular function, severe atrioventricular regurgitation and enteropathy should be counseled against pregnancy.14 Women with a systemic right ventricle (following Mustard or Senning surgery or with congenitally corrected transposition of the great vessels) have a similar risk of cardiac complications (10-30%), and should be assessed before pregnancy. Pregnancy should be discouraged in the presence of severe right ventricular dysfunction or TR.14 In uncorrected cyanotic CHD without PH, 32% of pregnancies are associated with complications, most often HF. Fetal outcome is directly related to the mother's oxygen saturation at rest (≤85% saturation is associated with fetal survival of only 12%).46
Pulmonary hypertensionPH is associated with high maternal mortality, but advances in pulmonary vasodilator therapy have raised hopes of improvements in prognosis.47 Among types of PH, the best prognosis is seen with idiopathic PH under specific therapy, for which mortality is 9%.48 In this group, women with vasoreactive PH who are stable under calcium channel blocker therapy have a relatively good prognosis during pregnancy.49
Despite improvements in prognosis for women with PH in recent decades, this condition is still associated with high mortality, and is categorized as risk level IV in the WHO classification. No criteria have been agreed for identifying women with lower risk during pregnancy, and all women diagnosed with PH are advised not to become pregnant.14 If a woman decides to continue with the pregnancy, she should be referred to a center specializing in PH and followed by a multidisciplinary team. Pulmonary vasodilator therapy in use before pregnancy should be continued, except for endothelin receptor antagonists (bosentan, macitentan and ambrisentan), which are teratogenic and should be replaced by sildenafil and/or prostacyclin derivatives.
Peripartum cardiomyopathyIn 2010, the ESC's working group on PPCM proposed a simplified definition of this entity, as an idiopathic cardiomyopathy frequently manifested by HF secondary to LV systolic dysfunction (LVEF <45%) towards the end of pregnancy or in the months following delivery, where no other cause of HF is found.50 As there is as yet no specific examination for diagnosing PPCM, it is a diagnosis of exclusion and must be differentiated from existing heart failure decompensated by the hemodynamic changes underlying pregnancy. What little epidemiological information is available on this entity comes mainly from Nigeria, South Africa and Haiti, where its incidence is higher, and the US, where its incidence is increasing.51 In 2017, Sliwa et al. published data gathered between 2012 and 2016 in the EURObservational Research Programme52 demonstrating that PPCM occurs in women from all over the world, with different ethnic origins and socioeconomic conditions, but with very similar forms of presentation and course. Risk factors that have been identified are African-American descent, older maternal age, multifetal pregnancies and hypertensive disorders during pregnancy.51 Although its etiology remains unknown, various mechanisms have been suggested, such as low selenium levels, reactivation of latent viral infections, stress-activated cytokines, inflammation, autoimmune reactions, pathological response to hemodynamic stress and unbalanced oxidative stress.53 Recently a new potentially causal factor has been described, cleavage of prolactin to produce a 16-kDa N-terminal prolactin fragment (16K PRL), mediated by oxidative stress.54 The antiangiogenic effect of 16K PRL and of soluble fms-like tyrosine kinase-1 (sFlt-1), levels of which are also high in this condition, can change the balance of angiogenesis, leading to vascular damage and hence HF.53 The high incidence of PPCM in Africans and a family history in 16% of cases have suggested a possible genetic cause,55 but the mutations documented so far are associated with familial forms of cardiomyopathy.
The majority of patients admitted with PPCM have typical symptoms and signs of HF. Differential diagnosis should be made with other entities including myocarditis, pre-existing cardiomyopathy, valve disease and congenital heart disease. When presentation is with cardiogenic shock, myocardial infarction and pulmonary embolism should immediately be excluded.12 An ECG should be performed in all patients suspected of having PPCM, even though there is no specific electrocardiographic pattern, due to its high negative predictive value. N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels are usually high and can be used to exclude non-cardiac-related dyspnea, although it does not help differentiate PPCM from other cardiomyopathies. TTE should be performed as soon as possible in all cases of suspected PPCM, to exclude other heart disease and complications such as apical thrombus, and to obtain prognostic information. Although prognosis is more favorable in PPCM than in other cardiomyopathies, it is associated with significant mortality (<5-50%) and morbidity (PE, cardiogenic shock, arrhythmias and thromboembolic events).12 Mortality risk is higher with advanced maternal age, multiparity, severely impaired global systolic function, African-American race and late diagnosis.4 The proportion of patients who recover left ventricular function (LVEF ≥50%) varies according to the study (35-70%), but in most cases this occurs within six months of childbirth.51 Recent studies show that African-American race and lower LVEF and greater LV end-diastolic volume at diagnosis are associated with a lower probability of recovery.56 In subsequent pregnancies, women with persistent LVD are at greater risk (around 50%) of clinical deterioration than those with complete recovery of ventricular function, although in the latter there may be recurrence in another pregnancy (cardiac function worsens in around 20%, in 20-50% of whom this persists following delivery).57
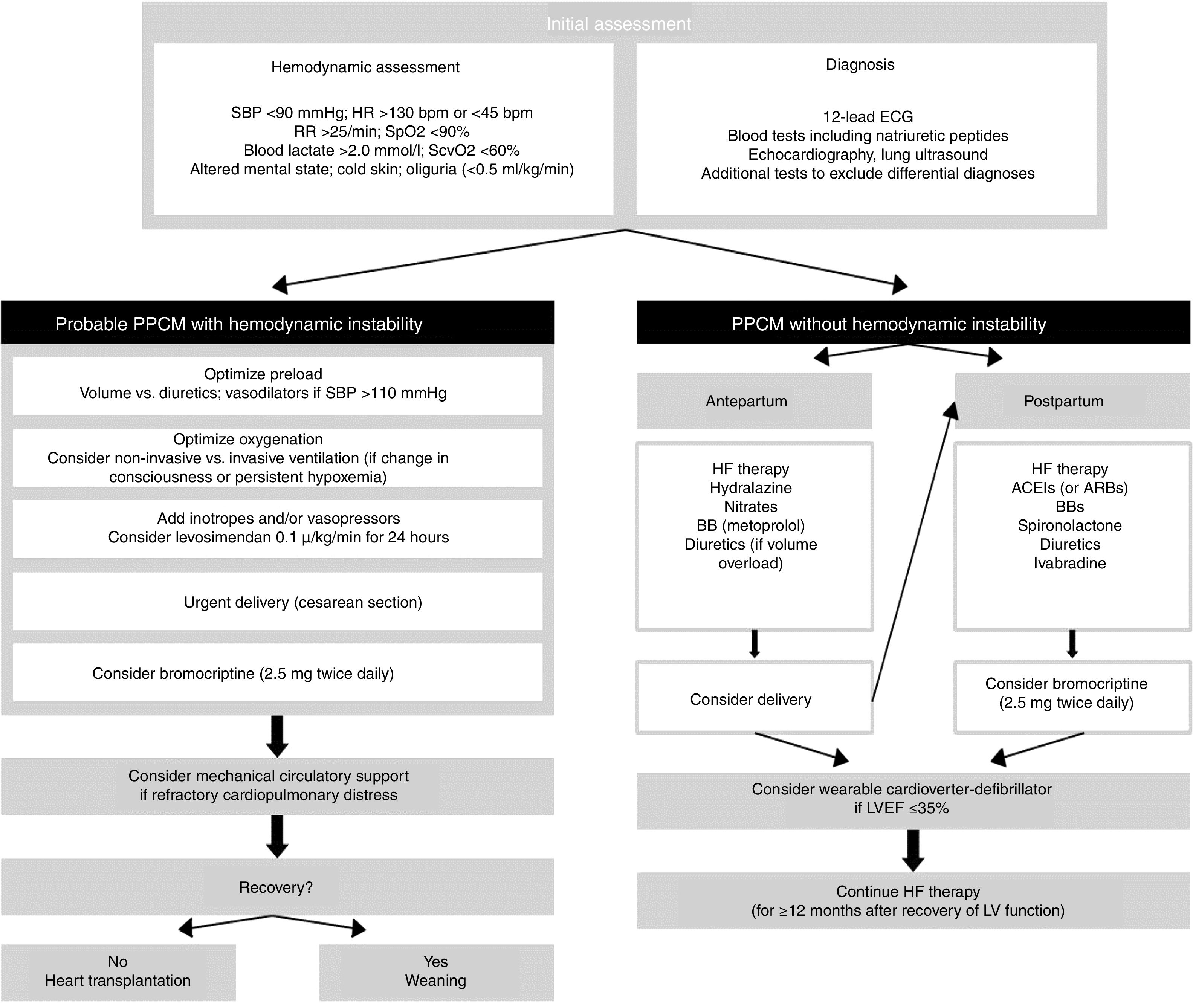
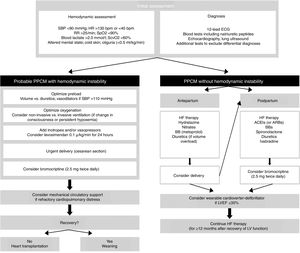
From a therapeutic standpoint, the approach to PPCM is similar to that of other causes of acute HF, taking care to avoid adverse effects on the fetus. Figure 1 shows a proposed treatment algorithm, according to the patient's hemodynamic stability. In hemodynamically unstable patients a rapid and systematic approach is essential in order to provide support and prevent target organ damage. This is one of the few situations in which an emergency cesarean section is indicated to treat the mother, with the aim of starting bromocriptine.58 Regarding inotropic support, levosimendan is preferred in these patients as it does not increase myocardial oxygen consumption, while catecholamines should be avoided. If levosimendan is unavailable, dobutamine is the inotrope of choice, and noradrenaline should be used as a vasopressor agent.12 Angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers and renin inhibitors are contraindicated during pregnancy due to their fetal toxicity. Alternatively, nitrates and hydralazine can be used to reduce pre- and afterload, respectively. After childbirth, ACEIs can be resumed, preferably captopril and enalapril during breastfeeding. While beta-blockers increase the risk of intrauterine growth restriction, they can be used in hemodynamically stable patients, preferably beta 1 selective beta-blockers such as metoprolol succinate. Mineralocorticoid receptor antagonists should be avoided in pregnancy and breastfeeding.14 Bromocriptine, in association with HF therapy, should be considered in view of its promising results in terms of recovery of LV systolic function and clinical improvement.59 In a German retrospective registry of PPCM, treatment with beta-blockers, ACEIs and bromocriptine (2.5 mg twice daily for two weeks followed by 2.5 mg once daily for six weeks) was associated with favorable outcomes.60 Anticoagulation with heparin should be started in patients with PPCM under bromocriptine and/or with LVEF ≤35% (during pregnancy and for at least eight weeks after delivery).61,62 Treatment to counteract ventricular remodeling should be continued for at least 12 months after recovery of LV dimensions and function. Although the main cause of death in PPCM is HF, one quarter of deaths occur due to ventricular arrhythmias, most in the first six months.63 The use of wearable cardioverter-defibrillators for six months after diagnosis of PPCM has been proposed for women with severe LV dysfunction, as a bridge to recovery of LV function or placement of an implantable cardioverter-defibrillator.63
Algorithm for management of patients with peripartum cardiomyopathy (adapted from Bauersachs et al.12). ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; BBs: beta-blockers; ECG: electrocardiogram; HF: heart failure; HR: heart rate; IV: invasive ventilation; LV: left ventricular; LVEF: left ventricular ejection fraction; PPCM: peripartum cardiomyopathy; RR: respiratory rate; SBP: systolic blood pressure; SpO2: peripheral oxygen saturation; SvcO2: central venous oxygen saturation.
The association between heart disease and pregnancy is increasingly prevalent. Pregnancy involves various adaptations which are not always tolerated by patients with existing heart disease. Assessment and monitoring of women with known or suspected heart disease who wish to become pregnant should therefore begin before pregnancy so that the individual's risk can be stratified and the measures to be taken can be scheduled in advance. Detection of a developing cardiovascular disorder must also be a priority in the monitoring of the pregnancy and if one is identified, the cardiology team should immediately be involved. It is increasingly common to have teams dedicated to dealing with cardiac disorders in pregnancy, an approach that is recommended, since it leads to better clinical outcomes.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Guimarães T, Magalhães A, Veiga A, et al. Cardiopatia e gravidez – o estado da arte. Rev Port Cardiol. 2019;38:373–383.