Serial echocardiographic assessment of left ventricular ejection fraction (LVEF) is the gold standard in screening for chemotherapy-induced cardiotoxicity (CIC). Measurement of myocardial deformation using speckle tracking enables more detailed assessment of myocardial contractility. The aim of this study was to determine the relationship between global and regional longitudinal strain and CIC.
MethodsThis was a prospective study of 158 breast cancer patients undergoing chemotherapy with anthracyclines with or without adjuvant trastuzumab who underwent serial monitoring by transthoracic echocardiography with assessment of myocardial deformation. CIC was defined as a decrease in LVEF to <53%. Global longitudinal strain (GLS) was estimated using EchoPAC BT12 software on a GE Vivid E9 cardiac ultrasound system. Patients were classified according to the 2015 ASE/EACVI criteria as having impaired myocardial deformation when GLS was reduced (less negative), with a cutoff of -18%.
ResultsDuring a mean follow-up of 5.4 months (1-48 months), the incidence of CIC was 18.9%. A decrease in GLS was observed during follow-up for the entire cohort (baseline GLS -20.1±3.5% vs. -18.7±3.4% at last follow-up assessment, p=0.001). A total of 97 patients (61.4%) were observed to have impaired myocardial deformation (GLS ≥18%) at some point during follow-up. This decrease was more significant in patients who eventually developed CIC (GLS -17.2±2.5%, p=0.02). On analysis of regional strain, impaired contractility was observed in the septal (6 out of 6) and anterior (2 out of 3) segments. Multivariate logistic regression showed that patients who developed impaired longitudinal strain had a 4.9-fold increased risk of developing CIC (odds ratio 4.88, confidence interval 1.32-18.0, p=0.017).
ConclusionsWorsening of myocardial deformation as assessed by speckle tracking is common in breast cancer patients undergoing chemotherapy, with predominantly septal and anterior wall involvement. Impaired myocardial deformation was independently associated with increased incidence of CIC.
A avaliação seriada da fração de ejeção ventricular esquerda (FEVE) é o padrão de referência na vigilância da cardiotoxicidade induzida por quimioterapia (CIT). A avaliação da deformação longitudinal com técnicas de speckle tracking permite uma avaliação mais detalhada da contractilidade miocárdica. O objetivo deste estudo foi avaliar a relação entre a deformação longitudinal global (GLS) e regional e a incidência de CIT.
MétodosForam incluídos 158 pacientes num estudo prospetivo observacional de doentes com neoplasia da mama submetidos a quimioterapia com antraciclinas, com ou sem terapêutica adjuvante com trastuzumab. Foi efetuada uma monitorização ecocardiográfica seriada de parâmetros de função sistólica e diastólica, incluindo a avaliação da deformação miocárdica longitudinal bidimensional. A incidência de CIT foi definida como uma diminuição da FEVE para valor<53%, de acordo com o documento de consenso da Sociedade Europeia de Imagiologia Cardiovascular (EACVI). Os doentes foram classificados como tendo deterioração da contractilidade miocárdica para um valor de GLS menos negativo que -18%, de acordo com os critérios da EACVI.
ResultadosDurante um período de seguimento médio de 5,4 meses (1-48 meses), a incidência de CIT foi de 18,9%. Na população global observou-se uma deterioração significativa do GLS durante o tratamento com quimioterapia (-20,1±3,5% versus 18,7±3,4%, p=0,001), com compromisso do GLS detetado durante em algum período do seguimento em 61,4% dos doentes. Esta deterioração foi mais marcada no subgrupo de doentes com CIT (GLS: -17,2±2,5%, p=0,02). Na análise regional da deformação longitudinal, verificou-se compromisso da contractilidade envolvendo preferencialmente os segmentos septais (seis em seis) e parede anterior (dois em três). Por regressão logística multivariada, o compromisso do GLS esteve independentemente associado ao desenvolvimento de CIT (odds ratio 4,88, IC 1,32-18,0, p=0,017).
ConclusõesO compromisso da deformação longitudinal é frequente em doentes submetidos a quimioterapia e mostrou um padrão de distribuição predominante a nível septal e anterior. A degradação da GLS foi um preditor independente de CIT.
Anthracyclines, and more recently trastuzumab (a monoclonal antibody that interferes with the HER2 receptor), are routinely used in the treatment of breast cancer.1,2 However, it was soon recognized that these drugs can lead to left ventricular (LV) systolic dysfunction as assessed by LV ejection fraction (LVEF) and to heart failure.3–5 Chemotherapy-induced cardiotoxicity (CIC) is one of the main determinants of prognosis in breast cancer survivors,6,7 but only a small subgroup of patients exposed to chemotherapy agents will develop CIC, which has a reported incidence of 3-12% for anthracycline therapy alone and up to 28% when combined with trastuzumab.
Effective strategies are therefore required for the screening and early detection of CIC, focusing particularly on a better understanding of the mechanisms responsible for deterioration of myocardial contractility.
Speckle tracking, now available in a range of echocardiographic systems, enables assessment of the different components of myocardial deformation – longitudinal, radial and circumferential strain. Decreased global longitudinal strain (GLS) in patients with preserved LVEF is associated with adverse cardiovascular events in a variety of clinical contexts.8,9
The aim of this study was to analyze changes in myocardial contractility as assessed by regional and global longitudinal strain in patients undergoing chemotherapy for breast cancer. In addition, we sought to establish whether changes in myocardial contractility before a fall in LVEF could predict the development of CIC.
MethodsStudy designThis was a prospective observational study of patients with breast cancer undergoing chemotherapy with anthracyclines (≥240 mg/m2 of doxorubicin or equivalent), with or without adjuvant immunotherapy, between June 2011 and October 2015. All patients referred by the hospital's oncology department for echocardiographic assessment prior to beginning chemotherapy and monitored during treatment were included. Those with a personal history of heart disease or with significant findings on initial echocardiography (LVEF <53%, LV end-diastolic diameter >60 mm, LV wall thickness >12 mm, moderate or severe valve regurgitation, or congenital heart disease) were excluded. Demographic data, current anticancer therapy and echocardiographic parameters were recorded.
Echocardiographic acquisition and processingInitial and follow-up echocardiographic assessments were performed according to the clinical indications established by the oncologist. GE Vivid 9 or Vivid 7 ultrasound systems were used to acquire parasternal long- and short-axis views, as well as apical 4-, 2- and 3-chamber views. A subgroup of patients (n=99, 63.1%) also underwent tissue Doppler study. LVEF was calculated by Simpson's biplane method.
Longitudinal strain was analyzed off-line following acquisition of apical 3-, 2- and 4-chamber views, using a semi-automatic algorithm in the EchoPAC BT12 software, based on regional assessment of 18 segments, the mean of which was used to calculate GLS. Inter- and intra-observer variability in assessing longitudinal strain in patients treated with anthracyclines has been reported previously.10
DefinitionsCIC was defined as LVEF <53% in accordance with the criteria of the 2014 expert consensus for multimodality imaging evaluation of adult patients undergoing chemotherapy of the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI).11 GLS was defined as reduced (less negative) using the cutoff of -18% recommended by the ASE/EACVI for EchoPAC BT12 software.12
Statistical analysisChanges in LVEF and global and regional longitudinal strain were analyzed by comparing baseline with follow-up echocardiograms. When more than one follow-up echocardiogram had been performed, the values corresponding to the greatest variation in the study variables were used.
Logistic regression analysis was used to determine predictors of CIC, with the incidence of CIC during follow-up as the dependent variable and including demographic characteristics, baseline echocardiogram, anticancer therapy, and the presence or absence of impaired GLS during follow-up prior to the development of CIC as independent variables. Variables with values of p<0.05 on univariate analysis were included in the multivariate model.
Continuous variables with normal distribution were compared using the Student's t test, while categorical variables were compared using the chi-square test. A value of p<0.05 was considered statistically significant.
ResultsPopulation characteristics and echocardiographic follow-upA total of 165 women were assessed, seven of whom were excluded due to LV dysfunction on baseline echocardiography; the final study population thus comprised 158 patients. Mean follow-up was 5.4 months (1-48), with a total follow-up time of 71.1 person years. All patients had undergone at least two echocardiographic studies, with a mean of 3.6 exams at a mean interval of 107 days.
Mean age was 54.6±12.9 years; 33% of patients underwent immunotherapy and 16.5% received radiotherapy. Baseline echocardiographic variables are shown in Table 1. There were no deaths from cardiovascular cause.
Baseline characteristics.
| Age (years ± SD) | 54.6±12.9 | |
| Female, n (%) | 158 (100) | |
| Chemotherapy with anthracyclines, n (%) | 158 (100) | |
| Immunotherapy, n (%) | 52 (33) | |
| Radiotherapy, n (%) | 26 (16.5) | |
| Follow-up (months) | 5.4 (1-48) | |
| Death from cancer | 4 (2.5) | |
| Echocardiographic variables | Mean | SD |
| LV end-diastolic diameter (mm) | 48.5 | 6.3 |
| LV end-systolic diameter (mm) | 29.0 | 5.6 |
| Fractional shortening (%) | 40.1 | 9.2 |
| LVEF (%) | 62.0 | 8.4 |
| LV end-diastolic volume (ml) | 75.6 | 23 |
| LV end-systolic volume (ml) | 28.6 | 14.6 |
| Transmitral E-wave velocity (cm/s) | 75.7 | 20.6 |
| Transmitral A-wave velocity (cm/s) | 79.3 | 19.2 |
| E/A ratio | 0.99 | 0.33 |
| Septal S’ (cm/s) | 7.47 | 1.77 |
| Lateral S’ (cm/s) | 8.89 | 2.45 |
| Tricuspid S’ (cm/s) | 12.5 | 2.5 |
| E/e’ ratio | 9.2 | 2.9 |
LV: left ventricular; LVEF: left ventricular ejection fraction; SD: standard deviation.
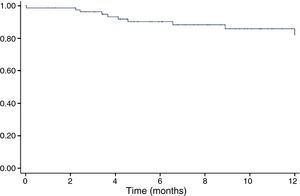
The incidence of CIC as defined above during follow-up in the study population was 18.9% (Figure 1), and was higher in the subgroup with adjuvant trastuzumab therapy (38.1% vs. 11.7%, p=0.001).
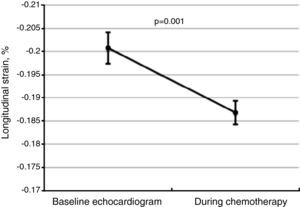
Global longitudinal strainThere was a significant worsening of GLS during follow-up, decreasing from a baseline value of -20.1±3.5% to -18.7±3.4% (Figure 2, p=0.001 on the Student's t test), and was more marked among patients who developed CIC (-17.2±2.5%, p=0.02). Impaired myocardial contractility was observed in a significant proportion of the total study population, with 61.4% of patients (n=97) presenting GLS below the cutoff of -18%.
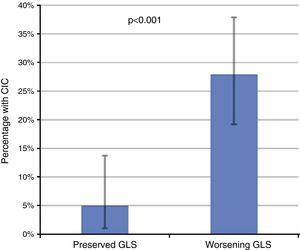
Of these 97 patients, 27 eventually developed CIC, while only three of the 61 patients with preserved GLS developed CIC (27.8% vs. 4.9%, p<0.001 on the chi-square test; Table 2, Figure 3). The mean period between evidence of worsening GLS and development of CIC was 101 days. Logistic regression analysis showed that worsening GLS was associated with a higher incidence of CIC (odds ratio [OR] 7.45, confidence interval [CI] 2.15-25.8, p=0.002); in multivariate analysis, the two independent variables associated with CIC were immunotherapy (OR 3.4, CI 1.21-8.2, p=0.018) and GLS below the cutoff of -18% (OR 4.88, CI 1.32-18.0, p=0.017) (Table 3). Subgroup analysis excluding patients undergoing immunotherapy showed similar results for the risk of developing CIC in the presence of worsening GLS (OR 6.24, CI 1.32-29.3, p=0.020).
Development of chemotherapy-induced cardiotoxicity and impaired longitudinal strain during follow-up.
| Without CIC | With CIC | Total | |
|---|---|---|---|
| Preserved longitudinal strain | 58 (36.7) | 3 (1.9) | 61 (38.6) |
| Impaired longitudinal strain | 70 (44.3) | 27 (17.1) | 97 (61.4) |
| Total | 128 (81.0) | 30 (18.9) | 158 (100) |
CIC: chemotherapy-induced cardiotoxicity.
Incidence of chemotherapy-induced cardiotoxicity (CIC) according to the presence of decreased global longitudinal strain (GLS), showing that patients with worsening GLS during follow-up had a significantly higher incidence of CIC. The error bars represent the 95% confidence interval calculated by the binomial test.
Univariate and multivariate analysis of predictors of chemotherapy-induced cardiotoxicity.
| Univariate analysis | p | Multivariate analysis | p | |
|---|---|---|---|---|
| Age | OR 1.01 (CI 0.95-1.05) | 0.832 | ||
| Trastuzumab | OR 4.33 (CI 1.87-10.1) | 0.001 | OR 3.14 (CI 1.21-8.2) | 0.018 |
| Radiotherapy | OR 2.47 (CI 0.90-6.82) | 0.079 | ||
| End-diastolic volume | OR 0.99 (CI 0.97-1.02) | 0.665 | ||
| Baseline LVEF | OR 0.01 (CI 0.00-8.96) | 0.186 | ||
| Development of impaired GLS (>-18%) | OR 7.45 (CI 2.15-5.83) | 0.002 | OR 4.88 (CI 1.32-8.0) | 0.017 |
CI: confidence interval; CIC: chemotherapy-induced cardiotoxicity; GLS: global longitudinal strain; LVEF: left ventricular ejection fraction; OR: odds ratio.
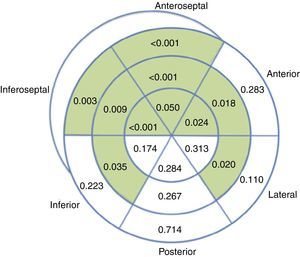
Overall, regional longitudinal strain was determined in 93.1% of the segments analyzed, with lower success rates in the laterobasal (86.1%) and posterobasal (81.4%) segments. Compared to baseline echocardiograms, exams performed during chemotherapy revealed impaired regional longitudinal strain in 10 out of 18 LV segments (Figure 4).
DiscussionThe main finding in this prospective study on echocardiographic assessment of longitudinal strain in breast cancer patients undergoing chemotherapy was an independent association between worsening GLS with preserved LVEF and the incidence of CIC. Patients with impaired GLS (below -18%) presented a high incidence of CIC (28.7%). Although preserved GLS did not preclude the development of CIC in our population, the finding does indicate a more favorable prognosis in terms of cardiovascular function, as these patients had a 4.9-fold lower probability of developing CIC.
As far as the authors are aware, this is the largest study to date assessing longitudinal strain in CIC.13 Our patient population was similar to those assessed in previous studies of cardiotoxicity in breast cancer patients,14,15 suggesting that our data could be used to identify patients at high risk of developing CIC.
A worsening of GLS was observed during chemotherapy with anthracyclines, with most patients presenting at least one echocardiographic exam showing GLS below the normal limit. This suggests the presence of subclinical myocardial dysfunction, even in patients who do not go on to develop CIC, as reported in other studies using tissue Doppler.16,17
Analysis of regional longitudinal strain appears feasible in this population, as it was successful in 93.1% of the segments assessed, a mean of 17 out of 18 segments per patient. Progressive worsening of regional strain was observed during chemotherapy, mainly involving the septal (6 out of 6) and anterior segments, sparing the posterior wall and most segments of the other walls. The preferential involvement of the septum has been reported previously in echocardiographic studies with visual assessment of regional wall motion,18 as well as in cardiac magnetic resonance imaging.19 This finding is also in agreement with a previous study that reported decreased longitudinal strain in septal, anteroseptal, anterolateral and apical segments in 27 patients undergoing chemotherapy with anthracyclines.15 While it needs to be confirmed in further studies, recognition of this pattern of septal and anterior involvement may prove useful when screening for chemotherapy-related cardiotoxicity.
The interest in longitudinal strain imaging and other techniques for the early detection of subclinical injury in this context is heightened by the fact that up to 58% of patients with CIC do not recover normal systolic function.5 Although multicenter studies20,21 and a recent meta-analysis13 support the use of speckle tracking echocardiography in screening for cardiotoxicity in patients treated with anthracyclines and trastuzumab, it is still not used routinely in clinical practice. The results of our study are in line with those of previous studies, and highlight the value of longitudinal strain for both assessment of subclinical cardiotoxicity and identification of patients at high risk of developing CIC.
The present study has certain limitations. The fact that the echocardiographic exams were performed based on clinical indication rather than at previously determined intervals may have led to gaps in the data. Mean follow-up was around six months, the usual period for echocardiographic monitoring during treatment with cardiotoxic drugs, but the duration of follow-up varied widely, ranging between one month and 48 months. For this reason, development of CIC at any point during follow-up was used as the event of interest, rather than at three or six months, since this is the relevant event in clinical practice, irrespective of the time elapsed between chemotherapy and CIC. Nevertheless, this approach may have led to underestimation of the incidence of CIC in patients with shorter follow-up in whom exposure to anthracyclines may not have reached a sufficiently high cumulative dose to cause LV dysfunction.
A further limitation is the fact that a third of patients presented HER2-positive breast cancer and were under adjuvant therapy with trastuzumab, which means the results cannot be generalized to those under anthracycline therapy alone. However, subgroup analysis of those under anthracycline monotherapy revealed similar results to those of the total population. Finally, no information was available on any pharmacological treatment used to prevent or treat cardiotoxicity, and this should be borne in mind when interpreting the results.
ConclusionsMyocardial contractility, as assessed by GLS, worsened in most patients undergoing chemotherapy for breast cancer, with predominantly septal and anterior wall involvement. Impaired GLS in patients with preserved LVEF was independently associated with increased incidence of CIC, and can thus be considered an earlier predictor of cardiotoxicity than merely calculating LVEF, as usually recommended.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Portugal G, Moura Branco L, Galrinho A, et al. Importância da deformação longitudinal na deteção da cardiotoxicidade induzida por quimioterapia e na identificação de padrões específicos de afetação segmentar. Rev Port Cardiol. 2017;36:9–15.