Pseudoaneurysm of the left ventricular outflow tract (LVOT) is a rare disease with high morbidity and mortality, resulting from left ventricular damage due to myocardial infarction, infective endocarditis or surgical trauma. A case of giant pseudoaneurysm of the LVOT, even more rarely reported in the literature, is described. The lesion was detected 12 years after aortic valve replacement for infective endocarditis in a young patient, a former intravenous drug user. As it is an uncommon disease, little is known about its clinical presentation and treatment.
O pseudoaneurisma da via de saída do ventrículo esquerdo (VSVE) é uma patologia rara decorrente de agressões sofridas pelo ventrículo esquerdo, como infarto agudo do miocárdio, endocardite infecciosa ou trauma cirúrgico, sendo uma afeção de alta morbimortalidade. Descreve-se um caso de pseudoaneurisma de VSVE gigante ainda mais raramente relatado na literatura. A lesão foi detetada 12 anos após a troca da válvula aórtica por endocardite infecciosa, num paciente jovem e ex-toxicodependente. Por ser uma patologia incomum, pouco se sabe sobre a sua apresentação clínica e respetivo tratamento.
computed tomography
extracorporeal circulation
left ventricular outflow tract
mitral-aortic intervalvular fibrosa
myocardial infarction
A 45-year-old man, an engineer and former intravenous drug user, with chronic hepatitis C, had undergone aortic mechanical valve replacement in May 1998 for infective endocarditis. Seven months later, he developed perivalvular dehiscence and was reoperated for implantation of a prosthetic patch in the ascending aorta. In 2009, he developed persistent atrial fibrillation, and attempts at chemical and electrical cardioversion were unsuccessful.
In 2010, he was admitted to our institution for diagnostic investigation of worsening dry cough, under therapy for pneumonia.
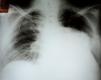
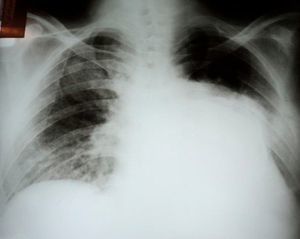
Initial diagnostic exams included simple chest X-ray, which revealed opacity in the base and middle third of the left hemithorax (Figure 1), 12-lead electrocardiogram, which showed atrial fibrillation and left ventricular overload, and laboratory tests, which were normal, with no anemia, leukocytosis or electrolytic changes.
Transesophageal echocardiography revealed left ventricular systolic dysfunction (ejection fraction 38%), moderate left atrial dilatation (50 mm) and significant ascending aortic dilatation (88 mm at its maximum diameter), with an image suggestive of flow between the aortic tube graft and the ascending aorta.
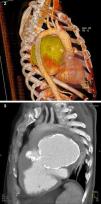
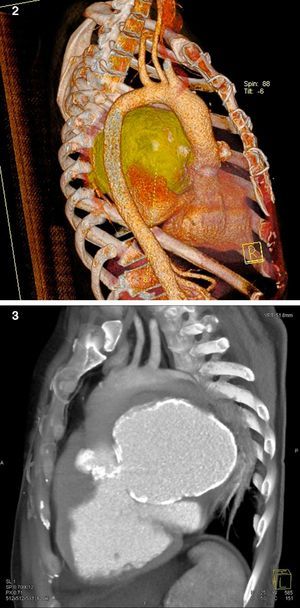
The patient then underwent computed tomography (CT) angiography, which showed a large pseudoaneurysm adjacent to the mitral-aortic intervalvular fibrosa (MAIVF), communicating mainly with the left ventricle (Figures 2 and 3), and compressing the pulmonary trunk, left pulmonary artery, left main bronchus, and the bronchovascular structures of the lingula and lower lobe of the left lung. There was also deviation of mediastinal structures to the left.
Cine coronary angiography revealed anatomically normal coronary arteries; ventriculography and aortography showed a normal ventricular chamber, with moderate inferolateral hypocontractility and reflux of contrast into a cavity suggestive of a giant pseudoaneurysm communicating with the left ventricle, with calcified walls but not obstructing ventricular ejection.
The patient remained hemodynamically stable during this period. Since the pseudoaneurysm was causing significant compression of neighboring structures, with clinical repercussions, the decision was made to intervene surgically.
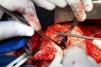
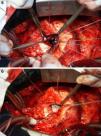
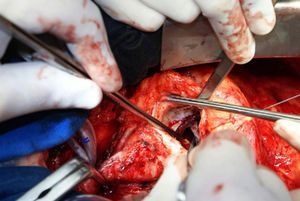
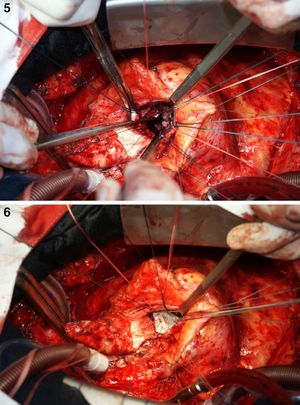
On October 5, 2010, the patient underwent cardiovascular surgery with extracorporeal circulation (ECC) and hypothermia at 23°C, with hyperkalemic blood cardioplegia at 5°C injected into the coronary ostia after aortic cross-clamping. When the aortic prosthesis was removed, an orifice approximately 1.5 cm in diameter was revealed (Figure 4), which was closed with a Dacron patch and a new 23-mm St. Jude aortic mechanical valve was implanted (Figures 5 and 6), followed by suturing of the aorta and re-warming to 37°C. The patient repeatedly developed ventricular fibrillation, requiring several 20-J shocks, and remained hemodynamically unstable, with significant hypocontractility and considerable difficulty in weaning from ECC. Venous infusion of noradrenaline and vasopressin was begun to maintain blood pressure levels.
Maximum doses of vasoactive drugs were required in the intensive care unit, and intra-aortic balloon pumping and monitoring by Swan-Ganz catheter were performed. The patient developed systemic inflammatory response syndrome, with hyperthermia, coagulopathy, and liver and kidney failure. On the third post-operative day, he was clinically stable; antibiotic therapy was increased and sedation discontinued. However, the following day, the patient was found to be neurologically unresponsive, and brain death was confirmed on October 11, 2010.
DiscussionPseudoaneurysm of the left ventricular outflow tract (LVOT) is a rare but highly lethal complication. It was first described in 1969 by Lewis et al.,1 who reported three cases of impending rupture in an autopsy series of 1228 patients with myocardial infarction (MI). In 1988, Savage et al. reported a case diagnosed antemortem, which was successfully treated, and coined the term pseudoaneurysm.2 Its precise incidence is unknown, but it is even less common than cardiac rupture after MI, which has an incidence of 2–4%.
LVOT pseudoaneurysm arises from incomplete myocardial rupture, the cavity being surrounded by cardiac muscle and remaining intact due to adhering pericardium or scar tissue in the left ventricular free wall. It mainly occurs after MI, but also following chest trauma, cardiac surgery (a third of cases)3 or infective endocarditis; it may also be due to congenital heart disease. Areas subjected to surgical manipulation are particularly vulnerable, due to dehiscence in the MAIVF.4,5 Its incidence is higher (although not significantly) in patients who have undergone aortic valve reoperation, compared to those operated only once (83% and 58%, respectively).
Valve endocarditis is the most common cause of dehiscence in the MAIVF and pseudoaneurysm formation. The fact that this region is poorly vascularized makes it more susceptible to infection. Contamination occurs either through contact with the aortic wall or through dissemination by the regurgitant jet to subaortic structures and the mitral valve anterior leaflet.
Congestive heart failure is the most common clinical manifestation, followed by chest pain, dyspnea and hemoptysis; in some cases sudden death can be the first symptom. Patients are asymptomatic at diagnosis in 12% of cases.4
Once formed, a pseudoaneurysm begins to exert additional stress on the aortic wall, which can lead to rupture. Rupture into the pericardium can cause cardiac tamponade, an eccentric mitral regurgitant jet tends to form in the atrium, and in the aorta a fistula occurs communicating with the ventricular chamber, all of which are associated with high morbidity and mortality. Pseudoaneurysms also predispose to embolization and infection. In some cases, the lesion remains intact and becomes chronic, appearing as a pulsatile cavity that expands in systole. There have also been reports of compression of the coronary arteries, causing ischemic symptoms; in these patients, the most common cause of death is heart failure or coronary disease, rather than myocardial rupture.6
Diagnostic methods include echocardiography, catheterization, CT, magnetic resonance imaging and angiography; while the latter is the gold standard exam, it is invasive and more costly.3 Three-dimensional echocardiography can also be used, although there are few studies on its efficacy in the diagnosis of pseudoaneurysms and their complications.
Since pseudoaneurysms are associated with high morbidity and mortality, with a high risk of serious and potentially fatal complications, most studies recommend surgical repair,2,7–9 without which the survival rate is low.7 Surgical mortality is now acceptable at 7–23% following technical advances, although there have been reports of spontaneous resolution of pseudoaneurysms.10 In clinically stable patients with chronic pseudoaneurysms of more than three months’ duration, some authors suggest non-invasive treatment with frequent follow-up, which reduces the risk of complications and mortality. Surgical intervention in these patients is recommended in the presence of complications such as tachyarrhythmia or recurrent embolism, if surgery is indicated for another reason, or when the diagnosis is made within three months of MI.6
We present a case of giant pseudoaneurysm of the LVOT, detected 12 years after aortic valve replacement for infective endocarditis in a young patient, a former intravenous drug user. As it is an uncommon disease, with few cases reported in the literature, little is known about its clinical presentation and treatment.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Acioli Pereira L, et al. Pseudoaneurisma gigante da via de saída do ventrículo esquerdo: uma patologia rara. Rev Port Cardiol. 2013. http://dx.doi.org/10.1016/j.repc.2012.11.006