Myxomas are the most common type of benign cardiac tumor. The most frequent clinical presentations are symptoms resulting from atrioventricular valve obstruction or systemic embolization. Coronary embolization is a rare, although real and potentially fatal, complication of cardiac myxomas. We present a case report and review of the literature on this disease association. A 57-year-old woman was admitted to our coronary care unit with a diagnosis of non-ST elevation acute myocardial infarction. Transthoracic echocardiography showed a large left atrial mass attached to the interatrial septum, coral-like and with a friable appearance, suggestive of myxoma. Coronary angiography revealed no significant lesions and the patient underwent surgical excision of the mass, which histological study showed to be compatible with myxoma. The postoperative period was uneventful and the patient is doing well, with no recurrence of myxoma.
Os mixomas são os tumores cardíacos benignos mais frequentes. Manifestam-se, habitualmente, por sintomas decorrentes da obstrução das válvulas auriculo-ventriculares ou de fenómenos de embolização. A embolização coronária é uma complicação rara dos mixomas cardíacos, mas real e potencialmente fatal. Apresentamos um caso clínico e a revisão da literatura relativamente a esta associação clínica. Trata-se de uma doente do sexo feminino, 57 anos de idade, internada na Unidade Coronária com o diagnóstico de enfarte agudo do miocárdio (EAM) sem elevação do ST. No ecocardiograma transtorácico observou-se uma volumosa massa auricular esquerda fixada ao septo interauricular, de estrutura coraliforme e aspeto friável, sugestiva de mixoma. A angiografia coronária não revelou lesões significativas e a doente foi submetida a cirurgia de excisão da referida massa, cujo exame histológico foi compatível com mixoma. O pós-operatório decorreu sem complicações e a doente mantém-se clinicamente bem e sem recorrência do mixoma.
Primary cardiac tumors are rare1; most are benign, and of these, around half are myxomas, often located in the left atrium.2
Clinical presentation is usually non-specific, with mainly constitutional symptoms, although there may also be symptoms resulting from atrioventricular valve obstruction or systemic embolization, and so early diagnosis is a challenge in clinical practice.
Coronary embolization is a rare, although real and potentially fatal, complication of cardiac myxomas. Early echocardiography in the context of acute myocardial infarction (AMI) is essential for diagnosis, together with urgent referral for cardiac surgery to resect the myxoma in order to avoid the potentially catastrophic consequences of further coronary or systemic embolization.
Case reportA 57-year-old woman, an ex-smoker with a history of non-insulin treated type 2 diabetes, dyslipidemia and bipolar disorder, went to the emergency department with epigastric pain and nausea of around a week's evolution, worsening the day before admission. No triggering, relieving or aggravating factors were identified, although her symptoms were difficult to characterize; she also reported generalized arthralgia for some months, mainly during the day and not mechanical in nature, but no chest pain, dizziness, syncope or symptoms of heart failure.
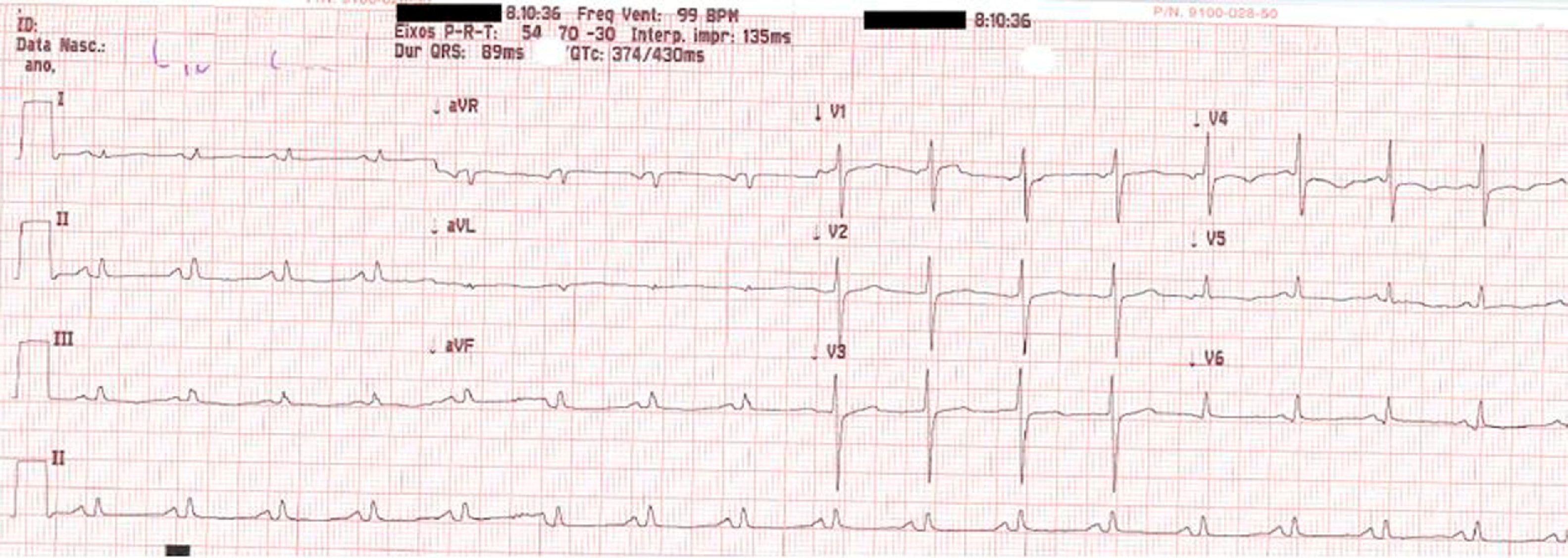
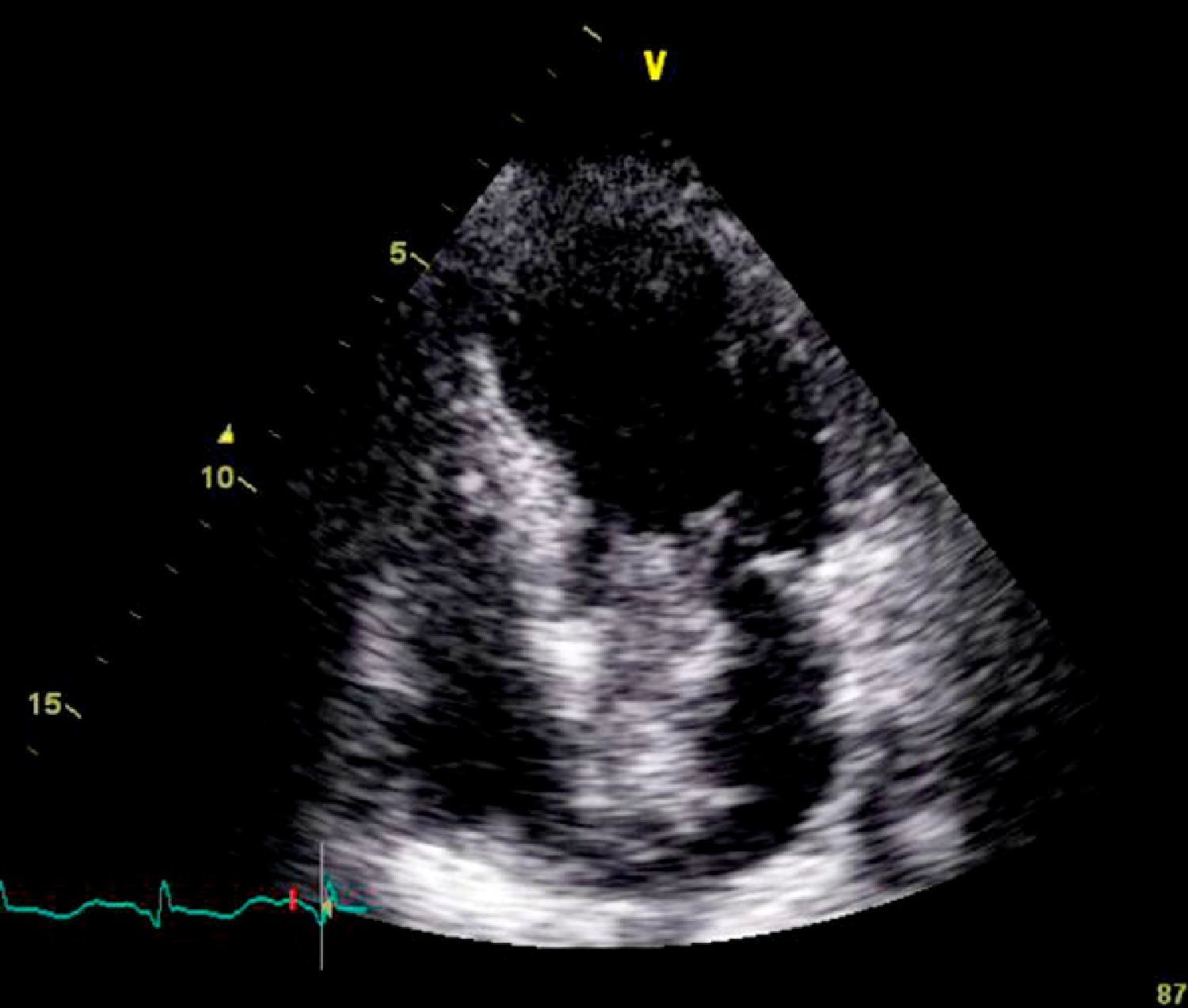
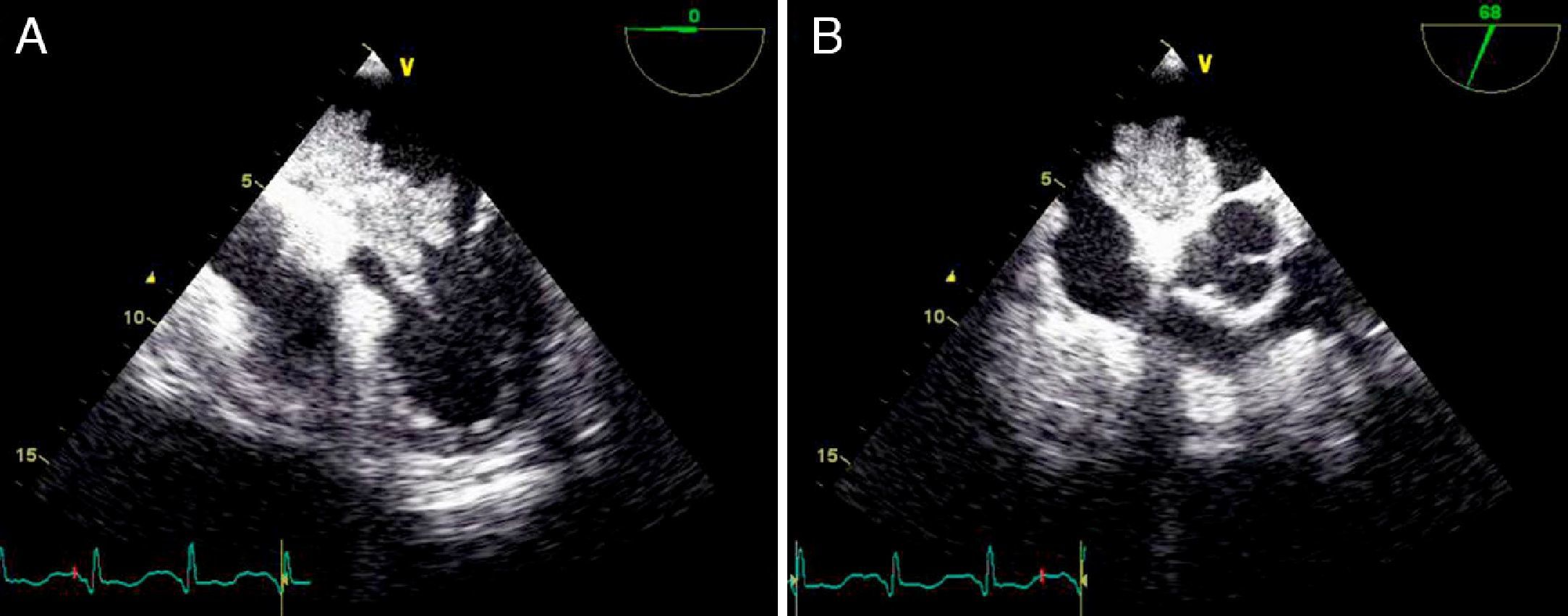
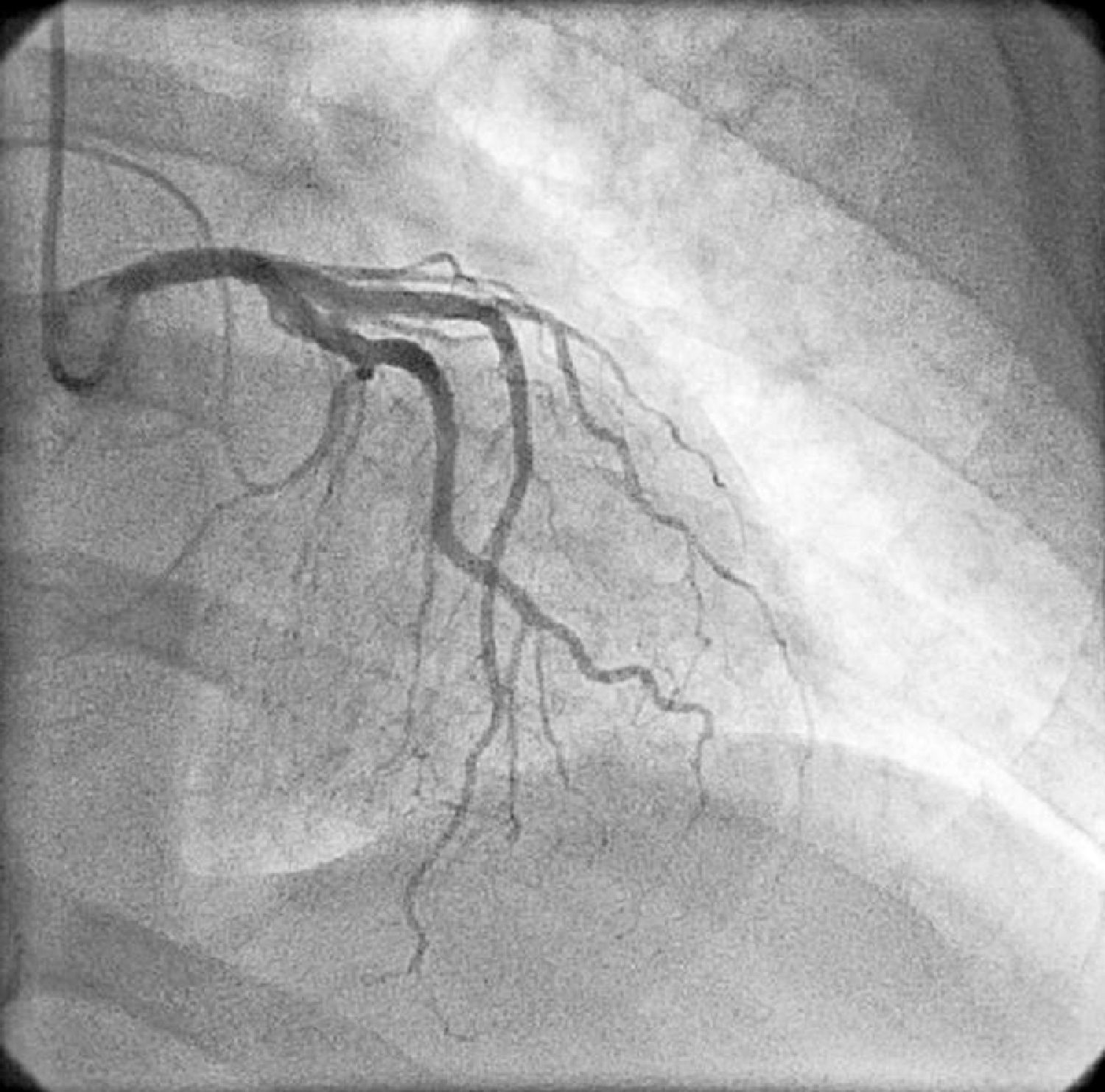
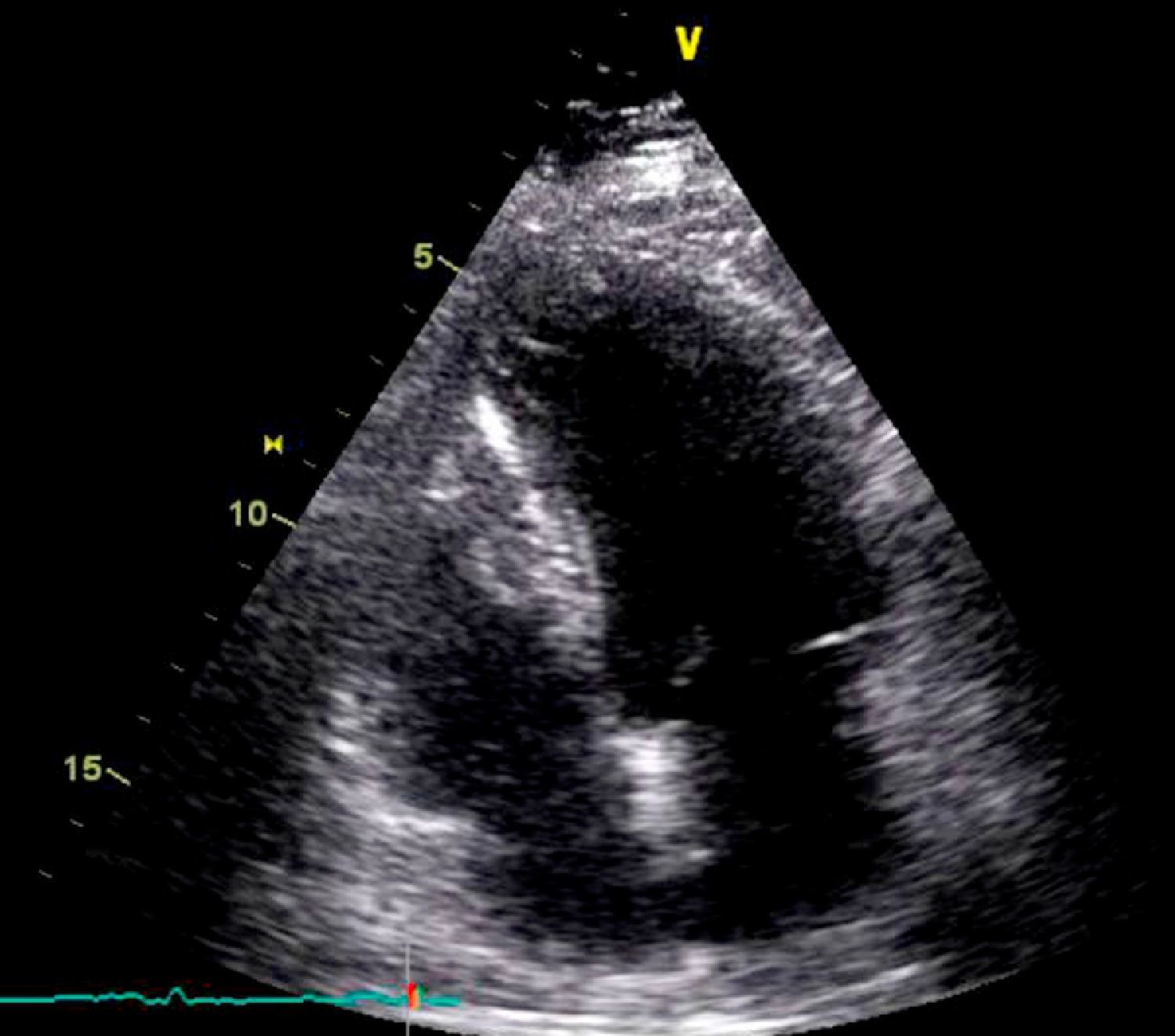
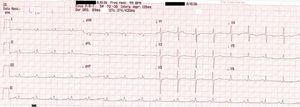
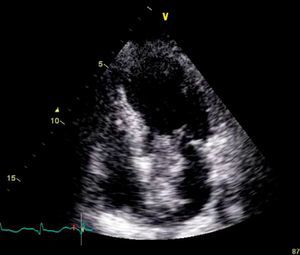
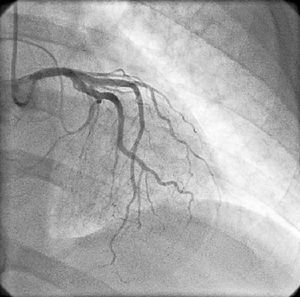
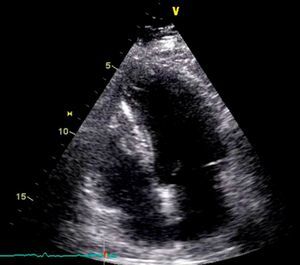
On physical examination she was hemodynamically stable, with no relevant alterations. The 12-lead electrocardiogram (ECG) showed sinus rhythm and ST-segment depression in V4, with negative T waves in V4-V5 and flattened T waves in V6 and frontal leads (Figure 1). Laboratory tests revealed hemoglobin 11.3g/dl, erythrocyte sedimentation rate 84mm, C-reactive protein 6.14mg/dl, troponin I 1.89ng/dl (rising slightly on the second assessment to 2.02ng/dl) and CK-MB 8.4ng/ml. She was therefore admitted to our coronary care unit with a diagnosis of non-ST elevation acute myocardial infarction. Transthoracic echocardiography showed moderate to severe left ventricular systolic dysfunction, with apical akinesia and hypokinesia of the mid-apical segments of the anterior and lateral walls and interventricular septum. As well as these wall motion abnormalities, a large left atrial mass was observed attached to the interatrial septum, coral-like and with a friable appearance, measuring 46mm on its longest axis, the most apical portion of which prolapsed into the left ventricle in diastole over the anterior leaflet of the mitral valve, without causing fixed obstruction to flow in the left ventricular outflow tract (Figure 2). Given the high suspicion of myxoma, transesophageal echocardiography was immediately performed (Figure 3), which confirmed the findings of transthoracic echocardiography. In view of the risk of imminent embolization, the cardiothoracic surgery department at our referral hospital was contacted and accepted our patient after coronary angiography, which revealed no significant lesions (Figure 4).
The patient underwent surgical excision of the left atrial mass, which macroscopically was a very large, extremely friable structure that fragmented when grasped with tweezers, the fragments forming a sphere 5cm in diameter, gelatinous and with hemorrhagic areas. Pathoanatomical study was compatible with myxoma.
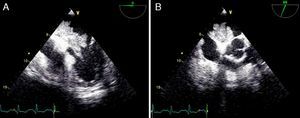
The postoperative period was uneventful, with normalization of left ventricular systolic function and almost complete reversal of the wall motion abnormalities. The patient is doing well, and to date no recurrence of myxoma has been detected on echocardiographic follow-up (Figure 5).
Discussion and ConclusionsMyxomas are the most common type of cardiac tumor, accounting for 50% of benign primary cardiac tumors.2 They are more frequent between the ages of 30 and 60 and in women.1 They are usually sporadic, although familial cases have been described, mostly multifocal, associated with the Carney complex.
Around 85% of myxomas are located in the left atrium; the attachment point is most often the fossa ovalis, followed by the right atrium (10%) and the ventricles (5%).3
Cardiac myxoma is rarely diagnosed on the basis of clinical history, physical examination or chest X-ray, since the signs and symptoms are not sufficiently specific to establish a firm diagnosis.4 Symptomatic cardiac myxoma may present with a variety of non-specific findings, depending on the size, location and mobility of the tumor. However, most cases fall within one of the three classic clinical presentations: obstructive, embolic or constitutional.
The obstructive pattern mimics mitral and/or tricuspid valve disease and results from atrioventricular valve obstruction. This can cause physical findings, particularly on auscultation, which often lead to an erroneous diagnosis of valve stenosis or regurgitation.
Constitutional or systemic manifestations include fatigue, fever, weight loss, arthralgia, myalgia, erythematous rash and laboratory findings such as anemia and elevated erythrocyte sedimentation rate, C-reactive protein and globulins. There is disagreement concerning their relationship with tumor size and location,1,4 but it has been suggested that they are related to the production and release of interleukin-6 by the tumor cells, or to intratumoral hemorrhage, microembolism, or the release of tumor fragments that trigger an immune response.3 Whatever their cause, these systemic manifestations are resolved by tumor resection.
With regard to embolic phenomena, particularly coronary embolization, around seventy cases have been reported of an association between AMI and cardiac myxoma. A review of these reports reveals that while systemic embolization from myxoma occurs in around a third of cases, the incidence of coronary embolization is only 0.06%, and hence AMI as the initial manifestation of myxoma is rare.5 Two possible explanations have been put forward for this low incidence: that the coronary ostia are oriented perpendicular to aortic flow, and that the opening of the aortic leaflets in systole protects the coronary ostia from emboli.5 However, it is suspected that this low incidence is an underestimation, since not all AMI patients undergo echocardiographic assessment, and there is a lack of published data on fatal events. It is thus important to perform echocardiographic assessment in all patients presenting with AMI before beginning therapy, since this is the only way to avoid the use of potentially harmful thrombolytic agents that increase the risk of embolization in the case of myxoma, either by lysis of accumulated thrombi or by hemorrhage and release of small fragments.6
Coronary angiography is valuable in cases of cardiac myxoma presenting with AMI to screen for coronary disease, especially in patients aged over 40 or with cardiovascular risk factors. Embolization to the anterior descending and circumflex arteries has been reported, but embolization to the right coronary artery is more common, possibly because of the orientation of the right coronary artery ostium relative to aortic flow. In a significant number of cases no coronary lesions are detected on angiography, which may be explained by the high rate of recanalization of coronary emboli from myxomas.6
Echocardiography remains the method of choice for diagnosis and morphological characterization of myxoma. Two patterns have been established by echocardiography: round, with a solid appearance and a firm surface, and polypoid, with an irregular outline and a friable surface. The incidence of systemic embolization is higher in those with an irregular and friable surface,4 as well as in polypoid tumors and those that prolapse into the ventricle.2
In the case presented, the left atrial myxoma was of the polypoid type with an irregular and highly friable surface, prolapsing through the mitral valve. Despite these characteristics and the tumor's large size, no obstructive symptoms or peripheral embolic phenomena had been documented prior to the current admission.
Once a diagnosis of myxoma is established, surgical resection is the only effective treatment and should be performed immediately, in view of the risk of imminent embolization. Long- and short-term prognosis is excellent and recurrence is rare, although six-monthly echocardiographic follow-up is recommended in all cases.1
With this case report, we aim to highlight the fact that a diagnosis as common in daily clinical practice as AMI may be a manifestation of such a rare entity as atrial myxoma, and that an embolic source should always be investigated in cases of AMI with angiographically normal coronaries. The absence of atherosclerotic coronary lesions or thrombi is compatible with the high rate of recanalization seen in emboli from myxomas, particularly those with a highly friable surface, as in our patient. We also stress the fundamental importance in this case of echocardiography, which was performed early and enabled a correct diagnosis and immediate referral for the only treatment that could prevent a potentially fatal outcome.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Marta L. Enfarte Agudo do Miocárdio como forma de apresentação de Mixoma gigante da aurícula esquerda. Rev Port Cardiol 2012. http://dx.doi.org/10.1016/j.repc.2012.04.013