The natriuretic peptides BNP and NT-proBNP are currently the most commonly used biomarkers in heart failure, but they have limitations. There is thus a need to identify new biomarkers that may prove useful, alone or in combination, for screening, diagnosis and prognosis.
Galectin-3 is a protein involved in a variety of cellular signaling pathways and is found in many tissues. Its expression is low in normal hearts but elevated in fibrotic hearts. Among other effects, it promotes fibroblast proliferation and collagen synthesis, contributing to the cardiac remodeling that is central to the development and progression of heart failure.
Heart failure associated with elevated galectin-3 (>17.8ng/ml) affects 30–50% of patients with chronic heart failure, and is a marker of worse prognosis, with higher rates of short-term rehospitalization and mortality.
It is thought that galectin-3 inhibition, or even genetic disruption, may reverse or delay disease progression.
Galectin-3 appears to have greater prognostic value than natriuretic peptides when assessed separately, however, when combined their prognostic value is even higher. Galectin-3, associated with BNP or NT-proBNP, may help improve the diagnosis and prognosis of heart failure.
Os péptidos natriuréticos BNP e NT-proBNP são atualmente os biomarcadores mais utilizados na insuficiência cardíaca, no entanto, permanecem algumas limitações. Isso justifica a necessidade de identificar novos biomarcadores que possam revelar vantagens, isoladamente ou em associação, no rastreio, diagnóstico e prognóstico. A Galectina-3 é uma proteína envolvida em diversas vias de sinalização celular. Pode ser encontrada em vários tecidos, sendo a sua expressão baixa no coração normal e elevada no coração fibrótico. Entre outros efeitos, é responsável pela proliferação de fibroblastos, promoção da síntese de colagénio, contribuindo para a remodelagem cardíaca que é determinante no desenvolvimento e progressão da insuficiência cardíaca.
A insuficiência cardíaca mediada pela Galectina-3 (>17,8 ng/mL) afeta 30-50% dos doentes com insuficiência cardíaca crónica, apresentando pior prognóstico, com maior taxa de reinternamento a curto prazo e mortalidade.
Pensa-se que através da inibição da ação da Galectina-3 ou mesmo através do knock-out genético se possa reverter ou promover um atraso na progressão desta doença.
Quando avaliados isoladamente, a Galectina-3 parece ter maior valor prognóstico do que os péptidos natriuréticos, no entanto, quando se combinam, o valor prognóstico é ainda superior. É possível admitir que a Galectina-3, associada ao BNP ou ao NT-proBNP, possa vir a ser considerada no futuro como uma alternativa que permita melhorar o diagnóstico e o prognóstico da insuficiência cardíaca.
N-acetyl-seryl-aspartyl-lysyl-proline
American Heart Association
area under the curve
carbohydrate recognition domain
Deventer-Alkmaar heart failure study
galectin-3
glomerular filtration rate
heart failure
left ventricular ejection fraction
N-terminal Pro-BNP Investigation of Dyspnea in the Emergency Department
receiver operating characteristic
transforming growth factor beta
Heart failure (HF) is associated with considerable mortality and morbidity. It is considered a 21st-century epidemic, and its prevalence is predicted to increase by 50–75% by 2030, rising with age. In Portugal, its prevalence is 1.36% between the ages of 20 and 50 and 16% over the age of 80.1,2
HF patients are frequently hospitalized (an average of twice a year in two-thirds), and 20–30% are readmitted within three months, incurring significant costs. Avoiding rehospitalizations would not only lead to considerable savings, but would also improve patients’ quality of life. There is thus a need for more and better diagnostic and prognostic methods.1,2
To improve the prognosis of those with HF, it is important not only to identify those with advanced HF, but also to be able to recognize those at highest risk for adverse events.3 There is thus a growing emphasis on the search for biomarkers that may be involved in the pathophysiology of the disease.4,5 Galectin-3 (Gal-3) levels are elevated even before HF is established,6 and it appears to identify HF patients at increased risk of early rehospitalization.7
Galectin-3The galectins are a family of animal lectins that contain a carbohydrate recognition domain (CRD) and bind to glycoconjugates containing N-acetyllactosamine.8
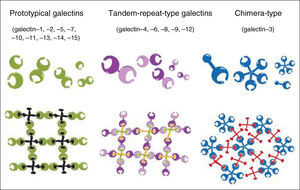
On the basis of their architecture, galectins are divided into three types: prototypical, tandem-repeat and chimera (Figure 1).8
Galectin family members and formation of galectin-glycan lattices. Reproduced with permission from Yang et al.8
Gal-3 is probably the best-studied member of the family. The only known chimera-type galectin, it has a molecular weight of 29–35 kDa and forms pentamers on binding to carbohydrates. It is found in a wide range of species and tissues.8
Gal-3 is most abundantly expressed in the lung, spleen, stomach, colon, adrenal gland, uterus and ovary, and also, albeit at a much lower level, in the kidney, heart, brain, pancreas and liver. However, under pathophysiological conditions, the level of expression of Gal-3 may change substantially,9 and is increased in cardiac fibrosis, liver cirrhosis, idiopathic pulmonary fibrosis, various cancers and renal fibrosis, the latter being one of the most important prognostic markers in HF.10,11 Gal-3 is a product of activated macrophages, with binding sites on cardiac fibroblasts, leading to an increase in myocardial collagen expression and interstitial fibrosis, transforming growth factor-beta (TGF-beta) activation, and subsequent left ventricular dysfunction. Accordingly, Gal-3 may play a pivotal role in the response to injury and inflammation in HF, including ventricular remodeling.6
Similar to other galectins, Gal-3 is localized primarily in the cytoplasm and, occasionally, in the nucleus and mitochondria. When secreted into the extracellular space, Gal-3 can interact with cell surface receptors and glycoproteins to initiate transmembrane signaling pathways for different cellular functions.6
Gal-3 has a variety of biological functions.8,10–13 It contributes to splicing of pre-mRNA. Outside the cell it induces apoptosis of T cells and monocytes, while intracellularly it has an antiapoptotic action. It mediates cell adhesion and aggregation, induces monocyte and macrophage migration, and promotes fibroblast proliferation and collagen synthesis. Gal-3 aids tumor growth, metastasis and angiogenesis, as well as accelerating re-epithelialization of corneal wounds and contributing to the growth and differentiation of B and T cells. It also has anti-inflammatory effects in asthma and modulates atherosclerosis and diabetes.
At the site of an injury, Gal-3 is secreted into the extracellular space, where it contributes to the fibrotic process by activating resting fibroblasts into matrix-producing fibroblasts. Fibroblast activation is characterized by increased expression of smooth muscle actin.12
Gal-3 not only affects the synthesis of new matrix components such as type I collagen; it also influences the degradation of extracellular matrix components through a set of tissue inhibitor metalloproteinases and matrix metalloproteinases.12
Galectin-3 in heart failureOnly the natriuretic peptides BNP and NT-proBNP are currently validated and recommended as biomarkers for diagnosis and prognosis in patients with HF.14,15 They respond rapidly to increased myocardial wall stress and their diagnostic value in the setting of chronic ambulatory and acute decompensated HF, particularly when the patient is dyspneic and the etiology is unclear, is well established.3,16
BNP and NT-proBNP are also used to monitor and optimize therapy. Lowering of levels over time in general correlates with improved clinical outcomes for these patients.16
However, although lower values of BNP or NT-proBNP exclude the presence of HF (with negative predictive value of 96% and 99%, respectively) and higher values have reasonably high positive predictive value to diagnose HF (79% and 76%, respectively), clinicians should be aware that plasma levels for both natriuretic peptides vary substantially over the day and elevated levels have been associated with a wide variety of cardiac (such as acute coronary syndrome, atrial fibrillation and myocarditis) and noncardiac causes (including advanced age, anemia, renal failure and pneumonia).12,16,17
Besides myocardial wall stress, other mechanisms play an important part in HF, including inflammation and pathways that regulate cardiac contractility. However, these mechanisms do not affect BNP and NT-proBNP levels, and there is thus a need to find new biomarkers that are linked to these processes.
Recent studies, including the Deventer-Alkmaar heart failure study (DEAL-HF) and Coordinating Study evaluating Outcomes of Advising and Counseling in Heart failure (COACH), identified Gal-3 as a biomarker of fibrosis and inflammation. These pathophysiological processes are involved in the cardiac remodeling that is central to the development and progression of HF. Since remodeling is a factor in worse prognosis in HF patients, reversing or slowing its progression is seen as an important therapeutic goal.10,12
Elevated plasma levels of Gal-3 in patients with acute and chronic HF have been consistently associated with adverse outcome.18
In subanalyses of the DEAL-HF, COACH, N-terminal Pro-BNP Investigation of Dyspnea in the Emergency Department (PRIDE) and Prevention of Renal and Vascular End-stage Disease (PREVEND) trials, higher Gal-3 levels were associated with older age,6,10,18–20 female gender (suggesting that regulation of Gal-3 is modulated by sex hormones)10,19, renal dysfunction as measured by glomerular filtration rate (GFR),6,10,19,20 cardiovascular risk factors (atrial fibrillation, hypertension, dyslipidemia and obesity),18,19 and higher BNP/NT-proBNP levels.6,10,18–20
In the subanalyses of DEAL-HF and COACH, Gal-3 was an independent prognostic marker even after adjustment for age, gender and BNP/NT-proBNP levels.10,20,21 However, the COACH subanalysis showed that correction for GFR resulted in some loss, albeit very small, of the prognostic power of Gal-3, suggesting that some of its predictive power may be mediated via renal function.18
In the subanalyses of DEAL-HF and PRIDE, there was no statistically significant relation between Gal-3 levels and HF etiology or presentation (acute or chronic), or New York Heart Association class.3,6,10–22 However, the COACH subanalysis did show a statistically significant correlation between left ventricular ejection fraction (LVEF) and the predictive value of plasma Gal-3, an identical increase in Gal-3 levels representing a much stronger incremental risk of mortality or HF hospitalization in patients with preserved LVEF (>40%) than in those with depressed LVEF (≤40%). However, absolute Gal-3 levels did not differ between patients with preserved and depressed LVEF.18
A subanalysis of Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) appears to conflict with these findings; although it showed associations between Gal-3 levels and age, as well as with hospitalizations and mortality, no relation was seen with gender, LVEF or NT-proBNP, and Gal-3 was no longer a significant predictor after the inclusion of NT-proBNP. One possible reason is that the trial included only patients with diastolic dysfunction (LVEF <35%), and the above studies suggest that the prognostic power of Gal-3 is greater in patients with preserved LVEF.14
Gal-3 levels increase with age, as do those of most cardiovascular biomarkers; preliminary data in octogenarians showed that Gal-3 levels were often higher than 20 ng/ml in patients without HF. It is therefore postulated that individual Gal-3 levels should be considered with respect to the age of the subjects, and that reference ranges for different age-groups are needed.19
Galectin-3 in heart failure screeningInflammation is a prerequisite for scar formation and tissue healing following injury. However, when inflammation becomes sustained it can lead to the formation of extensive scar tissue, resulting ultimately in complete organ failure, in this case HF.12 It is thought that Gal-3 promotes this diffuse fibrosis.22
In a study on rats, myocardial biopsies were obtained before overt HF had developed and those that later developed severe cardiac fibrosis and HF had the highest Gal-3 levels at the time of the biopsy.12 Sharma et al. also investigated the direct cardiac effects of Gal-3 by infusing it into the pericardial space of healthy rats. Development of cardiac remodeling was seen, with a significant decline in LVEF (Table 1) and increased collagen expression with a skewing of the type I to type III collagen ratio, an increase being seen in type I, which is less elastic than type III. This phenomenon may help explain the impaired left ventricular relaxation often seen in HF.
Comparison of echocardiographic changes after intrapericardial infusion of placebo or galectin-3.
| LVEF (%) | Placebo | Galectin-3 |
|---|---|---|
| Baseline | 67.3±2.5 | 66.1±1.6 |
| Post-infusion (4 weeks) | 66.0±0.4 | 51.8±3.1 |
LVEF: left ventricular ejection fraction. Adapted with permission from23.
As Gal-3 is overexpressed well before the transition to overt HF, this biomarker may in future help identify patients at risk of developing HF.9,23
Galectin-3 in heart failure diagnosisHenderson et al. observed that depletion of macrophages significantly reduced myofibroblast activation and decreased fibrosis, and concluded that macrophages are the main cells involved in the development of fibrosis.12 Since Gal-3 is a product of activated macrophages, it can be assumed that this biomarker has a direct effect on myocardial fibrosis and the development of HF.15
Gal-3 expression is low in normal hearts, but levels increase rapidly as HF progresses.12,24 HF associated with elevated Gal-3, i.e. that is significantly worsened by the presence of Gal-3 in cardiac tissue, affects 30–50% of patients with chronic ambulatory HF and two-thirds of those hospitalized for HF.22
However, although Gal-3 is involved in the processes that lead to HF, it is of less diagnostic value than BNP/NT-proBNP.15
Galectin-3 in monitoring and follow-up of heart failureAs pointed out above, the fact that Gal-3 levels rise before clinical manifestations of HF makes it a potential biomarker for risk of progression from stage B HF (evidence of structural heart disease but without symptoms) to stage C (symptomatic) in the ACCF/AHA classification.15
The subanalysis of the COACH trial demonstrated that Gal-3 levels are very stable over a six-month period, indicating that serial measurements would not yield superior predictive value: receiver operating characteristic (ROC) analysis for baseline Gal-3 showed an area under the curve (AUC) of 0.67, while for six-month Gal-3 levels it was 0.66. This may be related to the fact that Gal-3 activation and deposition in the matrix is an irreversible process, which differs substantially from published observations on the natriuretic peptides that suggest that repeated measurement may increase diagnostic and prognostic yield and may be used to guide therapy in HF patients.18
Gal-3 levels appear to be less influenced by the severity of symptoms, and are not affected in HF decompensation, and as they also remain unchanged by standard HF therapy they should not be used to guide treatment.15,22
In the future, identification of patients with elevated Gal-3 (>17.8 ng/ml) may help in various clinical decisions, including scheduling of follow-up visits (which should be more frequent as such patients have a worse prognosis), referral to a specialist if the patient is being monitored only by a general practitioner, and when to discharge hospitalized patients. One day Gal-3 levels may even be the target of specific therapy.22
Galectin-3 in heart failure prognosisGal-3 has been identified as an independent marker of adverse outcome in patients with HF.18 Patients with Gal-3 levels >17.8 ng/ml are two to three times more likely to be rehospitalized for HF within 30, 60, 90 and 120 days after initial discharge, irrespective of clinical status at discharge, compared to those with lower levels (Table 2).7
Risk for rehospitalization in patients with heart failure and elevated or low galectin-3 levels.
| Time after discharge | p | Percentage of patients rehospitalized for HF (galectin-3 ≤17.8 ng/ml) | Percentage of patients rehospitalized for HF (galectin-3 >17.8 ng/ml) |
|---|---|---|---|
| 30 days | 0.003 | 3.0% | 7.3% |
| 60 days | 0.001 | 4.5% | 10.0% |
| 90 days | <0.001 | 5.5% | 13.6% |
| 120 days | <0.001 | 7.3% | 15.8% |
HF: heart failure. Adapted with permission from Meijers et al.7
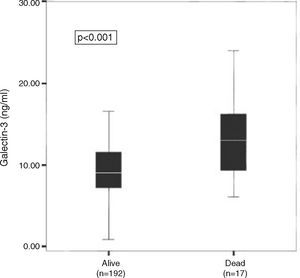
In the PRIDE subanalysis, Gal-3 levels in individuals who died within 60 days (Figure 2), one year (Figure 3A) and four years (Figure 3B) were significantly higher than in those who survived during those periods.3,6,7
Median galectin-3 levels among heart failure patients who died (n=17) within 60 days and those who survived (n=192). Results from the PRIDE subanalysis. Reproduced with permission from van Kimmenade et al.3
Galectin-3 in patients who died at one year (A) and four years (B) out of all dyspneic patients (n=115). Results from the PRIDE subanalysis. Reproduced with permission from Shah et al.6
In the subanalysis of the DEAL-HF trial, patients who died (n=98) had higher levels of both NT-proBNP and Gal-3 levels than survivors, and patients with higher NT-proBNP and Gal-3 levels had a 1.5–2-fold higher risk of mortality than those in other categories. There was also an inverse relation between survival and quartiles of Gal-3, with the first quartile (Gal-3 <13.63 ng/ml) presenting a higher probability of survival than the fourth quartile (>21.62 ng/ml).10
These findings support the initial assumption that Gal-3 plays an important role in the underlying disease and that elevated levels are associated with disease progression and worse outcome in HF patients.12
In the subanalysis of PRIDE, comparison of the value of these biomarkers by ROC curve analysis for prognosis of acute HF at 60 days after discharge produced an AUC of 0.74 for Gal-3 and 0.67 for NT-proBNP, suggesting that Gal-3 has greater prognostic value than BNP/NT-proBNP.3,7
The substudy of the COACH trial also showed that Gal-3 is a better predictor of prognosis than BNP, with AUCs of 0.67 and 0.65, respectively, but in combination their predictive power was higher (AUC of 0.69) than either biomarker separately, suggesting that their prognostic value is synergistic.15,18
Galectin-3 in heart failure therapyLiu et al. demonstrated that infusion of Gal-3 in rat hearts caused myocardial fibrosis and cardiac remodeling, while co-infusion with the antifibrotic agent N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) counteracted this effect.11,12 Adult male rats were divided into four groups and received the following intrapericardial infusion for four weeks: (1) vehicle (saline, n=8); (2) Ac-SDKP (800 μg/kg/day, n=8); (3) Gal-3 (12 μg/day, n=7); and (4) Ac-SDKP+Gal-3 (n=7). The authors found that in the left ventricle, Gal-3 enhanced macrophage and mast cell infiltration, increased cardiac interstitial and perivascular fibrosis, caused cardiac hypertrophy, and increased TGF-beta expression and SMAD3 phosphorylation. Ac-SDKP partially or completely prevented these effects, and the authors concluded that Ac-SDKP prevents Gal-3-induced cardiac inflammation, fibrosis, hypertrophy, and dysfunction in rats, possibly via inhibition of the TGF-beta/SMAD3 signaling pathway.25
There is thus speculation that blocking the action of Gal-3 may slow the progression of HF and possibly reduce associated morbidity and mortality.11,12 Drugs that bind to the CRD of Gal-3, such as Ac-SDKP, may reduce cardiac fibrosis, and are candidates for the prevention and treatment of HF.26
Liu et al. also saw positive effects in HF prevention through genetic disruption of Gal-3, which attenuated the development of cardiac remodeling, associated with a decrease in fibrosis. These findings suggest that Gal-3 is indeed involved in the pathophysiology of HF.27
However, there have as yet been no studies on Gal-3 as a therapeutic target in humans, and it is not possible to draw firm conclusions on the basis of animal models. We shall have to wait for future studies to shed more light on the role of Gal-3 in the treatment of HF.
ConclusionsTo summarize, Gal-3 appears to have lower diagnostic value but higher prognostic value than the natriuretic peptides in HF, and is particularly useful in patients with preserved LVEF. The combination of BNP/NT-proBNP and Gal-3 may be useful in identifying patients at high short-term risk for rehospitalization or mortality, enabling such patients to be managed appropriately.
With regard to therapy, infusion of Ac-SDKP prevented Gal-3-induced cardiac inflammation, fibrosis, hypertrophy and dysfunction in rats, and genetic disruption of Gal-3 reduced cardiac remodeling. As these interventions act on the pathophysiology of the disease, they may in the future become valid alternative therapies for HF.
There have been few studies on Gal-3 to date, and its role in the management of HF remains to be clarified.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pereira AR, Menezes Falcão L. Galectina-3: indicador de prognóstico. Alvo de intervenção terapêutica? Rev Port Cardiol. 2015;34:201–208.









 PRIDE subanalysis. Reproduced with permission from van Kimmenade et al.3'/>
PRIDE subanalysis. Reproduced with permission from van Kimmenade et al.3'/> PRIDE subanalysis. Reproduced with permission from Shah et al.6'/>
PRIDE subanalysis. Reproduced with permission from Shah et al.6'/>

