A 46-year-old woman was admitted due to diplopia because of ophthalmoplegia, which improved with corticosteroid therapy. Eight days later, she was admitted with fulminant myocarditis in cardiogenic shock, with severe left ventricular dysfunction and frequent episodes of nonsustained ventricular tachycardia. As there was no clinical improvement, an endomyocardial biopsy was performed that revealed inflammatory infiltrate, vasculitis, and PCR positive for cytomegalovirus, Epstein–Barr virus, parvovirus B19 and enterovirus. Left ventricular function recovered with heart failure treatment and corticosteroids. Three months later, after progressive withdrawal of prednisolone, there was recurrence of myocarditis and left ventricular dysfunction, which was successfully treated by restarting corticosteroid therapy. One month later she was readmitted with fulminant myocarditis which again responded to steroids. She intermittently presented cutaneous purpura lesions. At this time the provisional diagnosis was vasculitis and she started monthly cycles of cyclophosphamide. Before the second cycle, she was admitted with pneumonia and ventricular dysfunction and died.
Mulher de 46 anos foi internada por diplopia devido a oftalmoplegia, que melhorou com corticoterapia. Oito dias depois, iniciou quadro de miocardite fulminante associada a choque cardiogénico por disfunção ventricular esquerda grave e episódios frequentes de taquicardia ventricular não mantida. Por não apresentar melhoria clínica, foi submetida a biópsia endomiocárdica que revelou infiltrado linfocitário, sinais de vasculite e PCR positiva para citomegalovírus, vírus Epstein-Barr, enterovírus e parvovírus. Após tratamento da insuficiência cardíaca e corticoterapia, recuperou a função ventricular. Três meses depois e após a suspensão de prednisolona, teve recorrência da miocardite com disfunção ventricular, tratada com sucesso após reinício de corticóides. Um mês depois, foi reinternada com o mesmo quadro que respondeu ao aumento da dose de corticóides. Apresentou intermitentemente lesões cutâneas tipo púrpura. Colocou-se a hipótese de vasculite e iniciou tratamento com ciclos mensais de ciclofosfamida. Antes do segundo ciclo, foi internada com pneumonia associada a disfunção ventricular e faleceu.
According to the Dallas criteria, myocarditis is characterized as an inflammatory infiltrate of the myocardium. The most common cause is viral infection; less often it can be secondary to a non-viral infection, hypersensitivity to drugs or toxins, autoimmune disease, giant cell myocarditis or sarcoidosis.1 Clinical manifestations range from asymptomatic electrocardiographic (ECG) alterations, through non-specific systemic symptoms such as fever, myalgia, palpitations or exertional dyspnea, to cardiogenic shock and sudden death.2 In 1991, Lieberman et al.3 proposed a clinicopathologic description that classified myocarditis as fulminant, subacute, chronic active or chronic persistent. The clinical diversity of myocarditis makes it impossible to determine its real incidence, but it is estimated to cause 8.6–12% of sudden deaths in young adults and 9% of cases of dilated cardiomyopathy.1,2 Its prognosis and treatment depend on the underlying etiology and the clinical and hemodynamic repercussions.
Case reportA 46-year-old woman, working in air traffic control, with a history of smoking and right peripheral facial paralysis 15 years previously (with complete recovery), was admitted in June 2009 to the neurology department of Faro Hospital due to horizontal diplopia of sudden onset. She developed limited eye movement and bilateral ptosis but with preserved pupillary reflexes. Etiologic study included magnetic resonance imaging (MRI) of the cranium and orbits, electromyogram, and analysis of cerebrospinal fluid, including serology for Epstein–Barr virus (EBV), cytomegalovirus (CMV), herpes simplex 1 and HIV 1 and 2, and VDRL, none of which showed abnormalities. The chest CT was also normal and excluded thymoma. Immunologic study for ANA, ANCA, ECA and rheumatoid factor was negative, but was positive for skeletal muscle antibodies. She was treated with three 1-g methylprednisolone pulses and she was discharged two weeks after admission, with only right eye ptosis.
The provisional diagnoses were rapidly progressive external ophthalmoplegia, ocular myasthenia and mitochondrial myopathy. During this hospitalization, ECG, transthoracic echocardiography (TTE) and troponin I and BNP measurement were not performed.
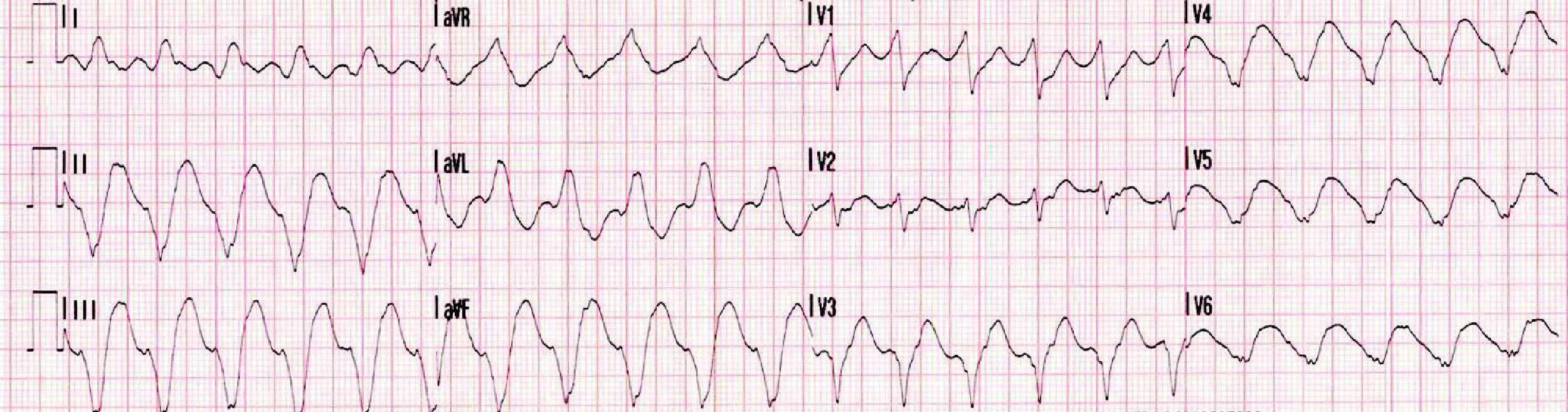
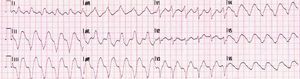
Eight days after discharge she was admitted to the cardiology department of the same hospital with fulminant myocarditis in cardiogenic shock and with frequent episodes of nonsustained ventricular tachycardia (VT) (Figure 1).
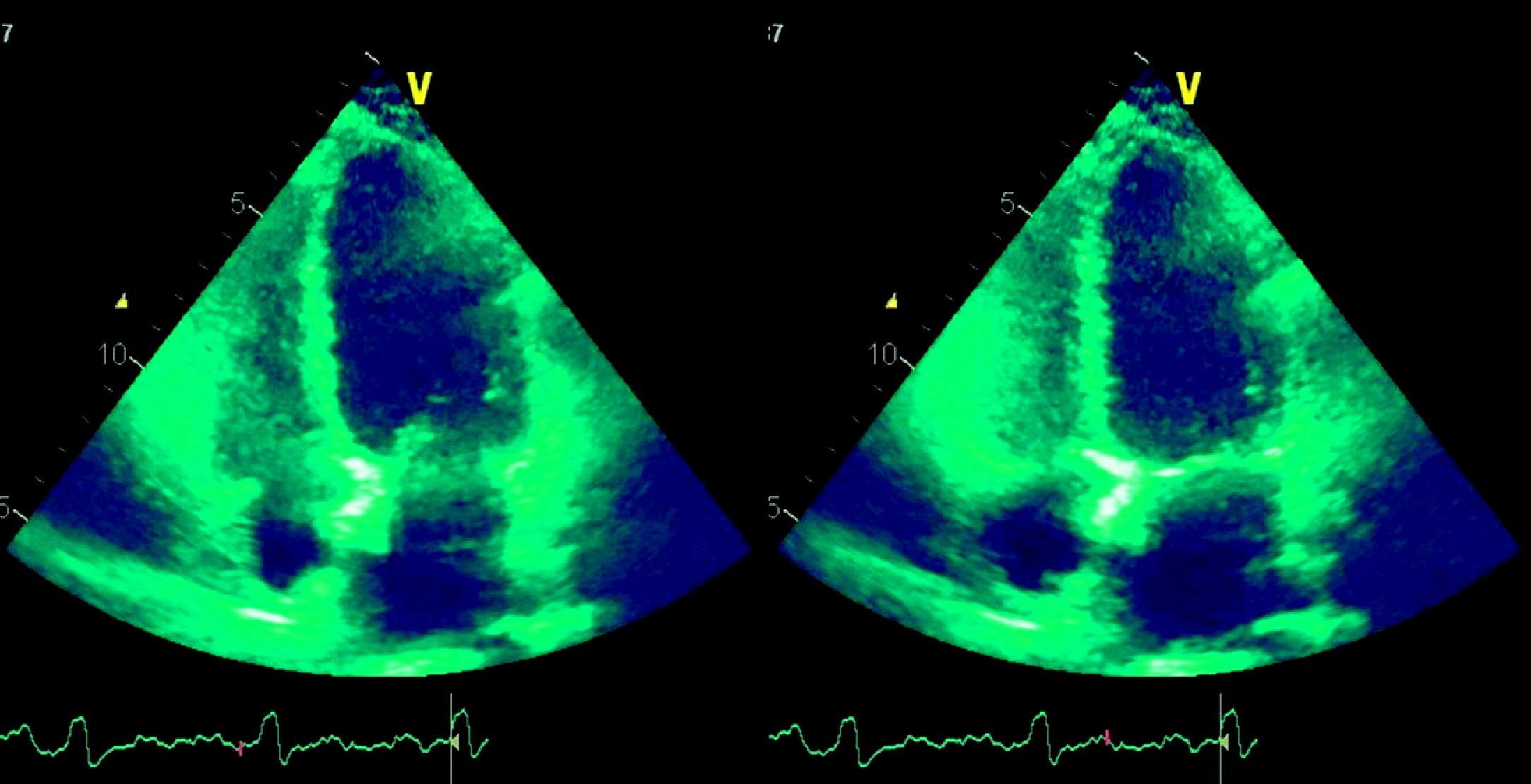
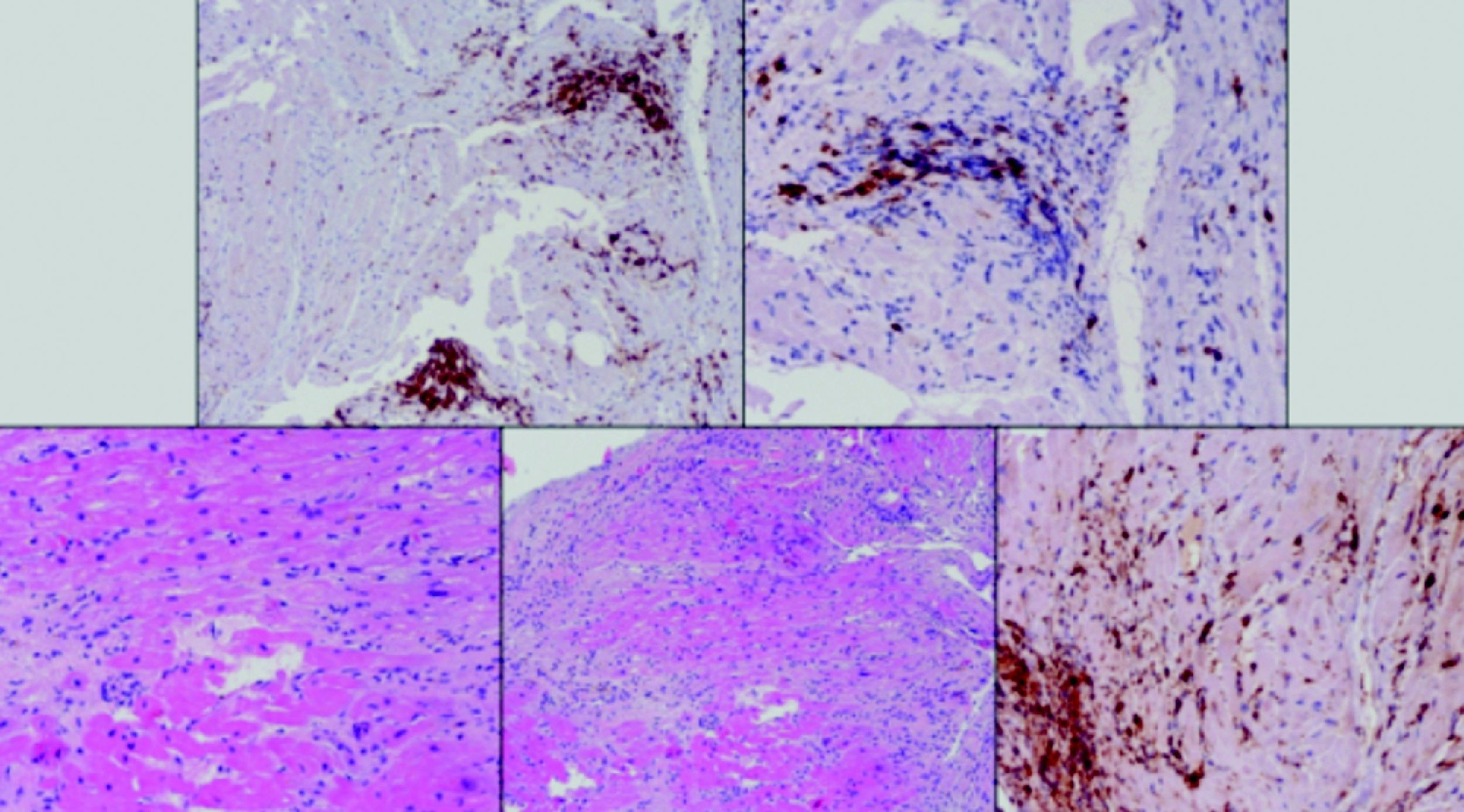
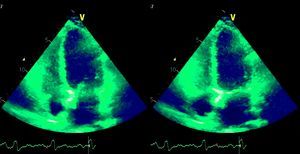
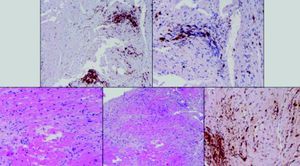
Laboratory test results included troponin I 12ng/ml (normal <0.1), BNP 2635pg/ml (normal <100) and CRP 76mg/dl (normal <5). The ECG showed sinus rhythm with incomplete right bundle branch block and symmetric negative T waves in V1–V6. TTE (Figure 2) revealed nondilated chambers, severe left ventricular (LV) dysfunction (LV ejection fraction [LVEF] 25%) with hypokinesia of multiple segments, and no pericardial effusion. Corticosteroid therapy was restarted (IV prednisolone 1mg/kg). On the fifth day, although no longer under IV inotropic support, she continued to present dyspnea at rest and frequent episodes of nonsustained VT and was therefore transferred to the advanced heart failure care unit of Coimbra University Hospitals with suspected giant cell myocarditis (GCM). Cardiac catheterization showed no angiographically significant coronary lesions; endomyocardial biopsy (EMB) of five specimens (Figure 3) revealed infiltrations of T lymphocytes, macrophages and CD20-positive B lymphocytes and signs of vasculitis, but did not detect multinucleated giant cells. Polymerase chain reaction analysis of the biopsy specimens was positive for parvovirus B19, enterovirus, EBV and CMV.
Serology for CMV, herpes simplex and EBV was positive for IgG but negative for IgM, and was negative for HIV, hepatitis C virus, Borrelia and Coxiella. Tests for smooth muscle antibodies were positive but were negative for skeletal muscle antibodies, unlike the result during the first hospitalization. The patient showed gradual clinical improvement and recovery of LV function under treatment with prednisolone and was discharged one month later with a diagnosis of fulminant myocarditis of probable autoimmune origin.
She remained in NYHA functional class I until the end of October 2009, when three days after suspension of corticosteroid therapy for the purpose of a muscle biopsy, she was readmitted for recurrence of myocarditis, severe LV dysfunction, cardiogenic shock and renal and liver failure. Steroids were restarted, leading to clinical improvement and recovery of LV function, and she was discharged under heart failure treatment and oral prednisolone. She underwent muscle biopsy as an outpatient under prednisolone 50mg/day, which showed type II fiber atrophy, compatible with steroid-induced and/or disuse myopathy. Control TTE showed nondilated chambers and good LV global systolic function (LVEF 57%). In order to reduce steroid use, azathioprine 100mg/day was introduced.
In mid-December, coinciding with a reduction in prednisolone from 50 to 40mg/day, she was readmitted to the cardiology department with hemodynamically tolerated sustained VT, which was converted to sinus rhythm by amiodarone. TTE again showed severe LV dysfunction. A 50-mg IV prednisolone bolus was administered and the oral dose was increased to 1mg/kg/day, and the daily dose of azathioprine was increased to 150mg/day. Clinical improvement and recovery of LV function were observed and she was discharged under heart failure therapy, amiodarone 400mg/day, prednisolone 60mg/day and azathioprine 150mg/day, strontium ranelate, calcium carbonate, and cholecalciferol.
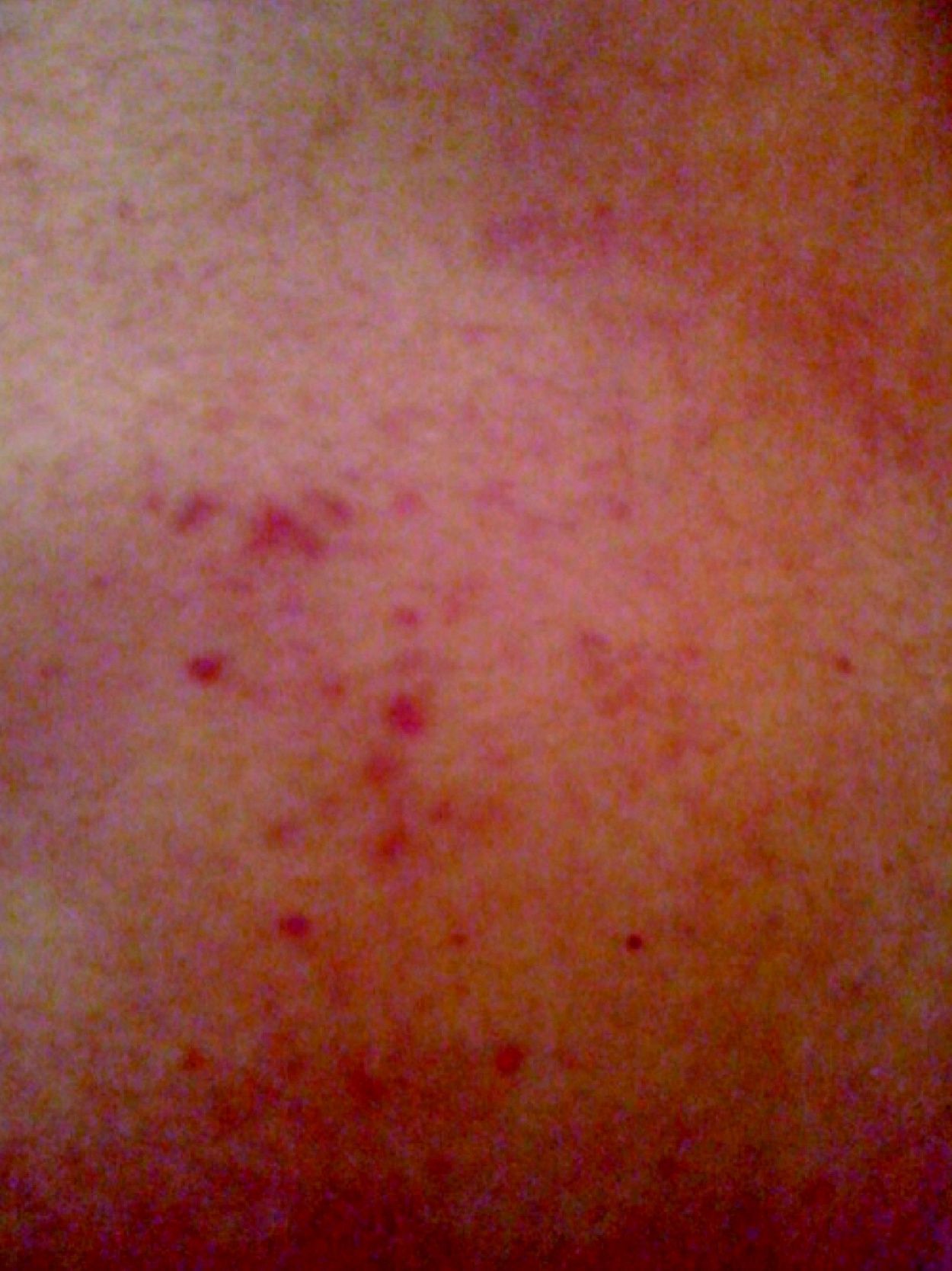
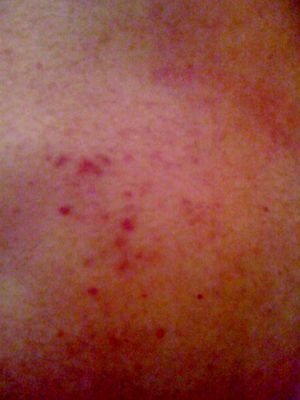
Since the second hospitalization (for fulminant myocarditis), the patient had intermittently presented cutaneous purpura lesions, mainly on the back (Figure 4).
The case was discussed with the internal medicine department and assessed in a center specializing in autoimmune diseases. Provisional diagnoses were vasculitis secondary to EBV infection or to dermatomyositis. The following therapeutic regimen was proposed: cyclophosphamide 0.7g/m2 monthly for six months, prednisolone (with indication for slow weaning 15 days after beginning cyclophosphamide, to a dose of 5–10mg/day), and restarting azathioprine after completing the treatment with cyclophosphamide.
The first cycle of cyclophosphamide was in January 2010, at which time TTE showed slightly depressed LV function (LVEF 43%) and mild mitral regurgitation.
In February 2010, when she was due to begin the second cycle, she was readmitted with dyspnea associated with fever and leukocytosis. Chest X-ray and chest CT showed, among other alterations, an alveolar infiltrate of ground-glass appearance, mainly in the right lung; an area of parenchymal condensation in the left inferior lobe; and interstitial fibrosis, particularly of the segmental and subsegmental septa. These findings were compatible with interstitial fibrosis associated with alveolitis. Given the suspicion of pneumonia, the patient was medicated with vancomycin, piperacillin-tazobactam and fluconazole. TTE showed moderate LV dysfunction. Two days after admission, invasive mechanical ventilation was required due to severe respiratory failure and hemodynamic instability. TEE revealed severe biventricular systolic dysfunction. On the third day she died from multiple organ failure. The family refused permission for an autopsy.
DiscussionFulminant myocarditis is an uncommon but serious form of myocarditis in which early supportive treatment is essential. The Dallas histopathologic criteria, published in 1986, were the first attempt to establish criteria for the diagnosis and classification of myocarditis based on EMB; they were limited by variability in interpretation of the specimen, lack of prognostic value (since neither symptoms nor clinical course are related to the extent of the lymphocyte infiltrate or fibrosis), and low sensitivity.1 Subsequently, Lieberman's clinicopathologic classification3 added clinical and echocardiographic criteria to the existing pathologic criteria, and Felker et al.4 included hemodynamic parameters. According to the latter two classifications the case presented corresponds to fulminant myocarditis, since the patient presented sudden-onset congestive heart failure with hemodynamic compromise and severe ventricular dysfunction with nondilated cardiac chambers, and signs of active myocarditis were later seen on EMB.
Viral infection is the most common cause of fulminant myocarditis. In the case presented here, four different viral genomes were found on EMB. In a series of “idiopathic” dilated cardiomyopathy, two or more viral genomes were identified in >25% of cases.2 As in most cases of myocarditis, viral serology in our patient did not match the genomic material identified on EMB; positive serology does not necessarily mean myocardial involvement. Although viral genomic material was detected, other aspects of this case led us to believe that it was in fact giant cell myocarditis, which is often associated with autoimmune disease: the initial hospitalization in the neurology department for external ophthalmoplegia which responded to steroids suggests an autoimmune etiology, while GCM usually presents as fulminant myocarditis and is often associated with VT.1,5 This ominous form of presentation was associated with hemodynamic instability and severe ventricular dysfunction in the case presented, despite supportive treatment.
Treatment of fulminant myocarditis is initially based on supportive treatment for left ventricular dysfunction, as stated in the European Society of Cardiology guidelines.8 The low incidence of fulminant myocarditis and the difficulty in establishing a definitive diagnosis have limited the number of randomized controlled trials to assess different therapeutic strategies. Various studies of immunosuppressants,6,7 including steroids, cyclosporin and azathioprine, intravenous immunoglobulins7 and interferon,2 have not shown benefits in treating myocarditis. Post-hoc analysis of a study of immunosuppressive therapy revealed that those who did not respond to the therapy showed a high prevalence of virus persistence but absent anticardiac antibodies.9 Although the role of such treatments in fulminant myocarditis is yet to be defined, immunosuppressive therapy may be beneficial in patients with anticardiac antibodies or with immunohistologic inflammation on EMB, while cases in which viral genomic material is detected on EMB may benefit from antiviral therapy.9 Although some studies1,2,6 do not recommend steroid therapy for all cases, it may be indicated in myocarditis secondary to autoimmune disease or in GCM,5 both of which were diagnostic possibilities in the case presented here, and the patient was therefore treated with prednisolone.
When supportive treatment with prednisolone failed to bring about clinical improvement, it was decided to perform EMB, which, while only carried out in a small percentage of patients with myocarditis, is the diagnostic gold standard. In the case presented it was a class I recommendation (level of evidence B), for unexplained, new-onset heart failure in addition to hemodynamic compromise.10 Moreover, in cases of fulminant myocarditis differential diagnosis between GCM and necrotizing eosinophilic myocarditis is essential.1
Although cardiac MRI was not performed in this case, it has high sensitivity and specificity to identify myocarditis,9 especially T2-weighted and gadolinium late enhancement images. Cardiac MRI is increasingly used for differential diagnosis with myocardial infarction (MI); patients with myocarditis exhibit diffuse or nodular delayed enhancement, subepicardial or intramyocardial, in a nonvascular distribution, while those with MI demonstrate subendocardial or transmural delayed enhancement in a segmental vascular distribution.11 Cardiac MRI also increases the sensitivity of EMB by identifying the best sites to perform the biopsy.
The fact that the diplopia leading to the initial hospitalization was secondary to ocular muscle involvement, together with cutaneous lesions, signs of vasculitis on EMB and increasing dependence on corticosteroid therapy after the third hospitalization, all led to a provisional diagnosis of vasculitis secondary to EBV infection or to dermatomyositis. Given the possibility of involvement of vital organs, it was decided to institute cyclophosphamide therapy. The etiology of this patient's disorder with systemic involvement, most likely a form of vasculitis, remains unclear; the impossibility of performing an autopsy limited further investigation of the case. Although this patient died, survival in fulminant myocarditis can reach 90%.1,2
Conflict of interestThere are no conflicts of interest.
Please cite article as: Faria, R; Miocardite Fulminante – a propósito de um caso clínico. Rev Port Cardiol 2012;31(7-8):503-507.