We aimed to study the feasibility and outcomes of ductal stenting in patients with duct-dependent pulmonary blood flow (PBF).
MethodsDuct-dependent hypoxic patients with confluent pulmonary artery (PA) branches were enrolled for ductal stenting and followed regularly.
ResultsSixty patients, with a median age of 12 (1-1095) days and weight of 2.8 (2.2-8.9) kg, were enrolled. Median right PA (RPA) and left PA (LPA) Z-scores were -1.23 (-10.54 to 2.81) and -0.96 (-8.03 to 3.0), respectively. Mean narrowest ductal diameter was 1.73±0.57 mm and length was 12.78±3.32 mm. Sixty-four stents with mean diameter of 4.21±0.32 mm and length of 14.34±3.44 mm were deployed in 59 patients. The procedure was unsuccessful in one. Post-stenting mean oxygen saturation (SO2) increased significantly from baseline of 68.88±7.47% to 90.43±6.04% (p<0001). Complications included pulmonary edema in one patient and acute stent occlusion in another. At a median follow-up of eight (2-14) months, mean SO2 (80.04±7.54%) was significantly higher than baseline (p<0.0001). Median RPA and LPA Z-scores, 0.56 (-2.89 to 3.29) and -0.02 (-2.81 to 3.86), respectively, were significantly higher than baseline. Six patients required re-interventions (shunt in three and angioplasty in three). Six patients died, three due to sepsis and another three with worsened cyanosis due to impaired PBF, probably due to ductal occlusion.
ConclusionDuctal stenting is an effective palliation in patients with duct-dependent PBF. It maintains adequate SO2 and promotes balanced PA growth at mid-term follow-up.
Este artigo tem como objetivo a avaliação da praticabilidade e dos resultados da implantação de stent em doentes com fluxo sanguíneo pulmonar dependente do canal arterial.
MétodosOs doentes hipoxémicos dependentes do canal arterial, com ramos da artéria pulmonar confluentes, foram admitidos para implantação de stent no canal arterial e sujeitos a seguimento regular.
ResultadosForam admitidos 60 doentes com a idade média de 12 dias (1-1095) e peso de 2,8kg (2,2-8,9kg). Os Z-scores médios da artéria pulmonar direita (APD) e da artéria pulmonar esquerda (APE) foram -1,23 (-10,54-2,81) e -0,96 (-8,03-3,0), respetivamente. A média do diâmetro do canal mais estreito foi de 1,73±0,57mm e o comprimento foi de 12,78±3,32mm. Sessenta e quarto stents com um diâmetro médio de 4,21±0,32mm e comprimento de 14,34±3,44mm foram colocados em 59 doentes. O procedimento não foi bem-sucedido num caso. A média de SO2 após a implantação dos stents aumentou significativamente o valor basal de 68,88±7,47% para 90,43±6,04% (p<0,001). Registaram-se complicações, tais como edema pulmonar num doente e oclusão aguda de stent noutro. Aos oito meses de seguimento (2-14 meses), a média de SO2 (80,04±7,54%) foi significativamente maior do que o valor basal (p<0,0001). A média dos Z-scores da APD e da APE, 0,56 (-2,89-3,29) e -0,02 (-2,81-3,86) respetivamente, foi significativamente maior do que os valores basais. Seis doentes necessitaram ser reintervencionados (shunt em três e angioplastia noutros três). Seis doentes morreram, três devido a sépsis e outros três com agravamento da cianose por limitações do fluxo sanguíneo pulmonar, provavelmente por oclusão do canal.
ConclusãoA implantação de stent é uma técnica paliativa efetiva em doentes com circulação pulmonar dependente de canal arterial. Mantem os níveis de SO2 adequados e promove o crescimento equilibrado da artéria pulmonar no seguimento a meio prazo.
Establishment of adequate pulmonary blood flow (PBF) in early life is essential for survival in children with duct-dependent pulmonary circulation. Surgical aortopulmonary shunt is a well-established palliation.1 Although shunting has been used in early childhood since the mid-1940s, it is still associated with significant morbidity and mortality2 due to various shunt-related complications, compromise of the subclavian artery and distortion of the pulmonary artery (PA) branches.1,3,4 Maintaining ductus arteriosus patency by percutaneous placement of a stent is an alternative palliation. It maintains adequate PBF and serves as a bridge to definitive surgery.5,6
ObjectivesWe aimed to study the feasibility and mid-term outcomes of ductal stenting in patients with duct-dependent PBF.
MethodsAfter clearance from our institutional ethics committee (UNMICRC/CARDIO/2013/17), duct-dependent hypoxic children were enrolled consecutively in a prospective study over two and a half years.
Inclusion criteria were hypoxia (transcutaneous arterial oxygen saturation [SO2] <75%) and duct-dependent pulmonary circulation, while exclusion criteria were non-confluent PA branches, non-restrictive large duct (narrowest diameter ≥2.5 mm), baseline SO2 >80%, prior shunt surgery, and requirement for some other procedure in addition to ductal stenting (e.g. pulmonary valve perforation).
Informed consent was obtained from patients’ guardians. Baseline SO2 and anthropometry were noted in all patients. On two-dimensional echocardiography (iE33 xMATRIX, Philips Healthcare, Andover, MA, USA), PA and ductal anatomy were evaluated in high parasternal and suprasternal views. Multidetector computed tomography (MDCT) was performed with a 128-slice scanner (Somatom Definition AS+, Siemens Healthcare, Malvern, PA, USA) to delineate these details as well. The origin, shape, diameter and tortuosity of the duct were evaluated. The PA branches were measured at prebranching levels. After calculation of body surface area with the Haycock formula, Z-scores were obtained using the Detroit data.
ProcedureProcedures were performed under general anesthesia or monitored anesthesia care. Femoral arterial, femoral venous or axillary arterial access was established with 4F to 6F sheaths (Cordis Corporation, Miami, FL, USA). The duct was approached in each patient using a Judkins right coronary (JR) catheter or internal mammary artery (IMA) catheter (Cordis Corporation, Miami, FL, USA). Selective arch and ductal angiograms were performed. Ductal origin, shape, course, tortuosity, diameter near the pulmonary end (commonly the narrowest diameter), diameter of ampulla, ductal length and PA anatomy (including stenosis if present) were evaluated. The duct was negotiated with a 0.014” COUGAR-XT floppy-tipped guidewire (Medtronic, Inc., Minneapolis, MN, USA). In some patients with an extremely tortuous duct this was replaced with an extra support wire, WHISPER-ES (Abbott Vascular, Santa Clara, CA, USA) to ease tracking of the stent-mounted balloon catheter over-the-wire. The wire was anchored distally in one of the PA branches. A Mullins sheath (Cook Inc., Bloomington, IN, USA) was used in several patients to support negotiation of the stent through a tortuous vertical duct. A bare-metal stent (BMS)-Driver (Medtronic, Inc., Minneapolis, MN, USA) was deployed in each patient (Figures 1 and 2). If there was a stenosis of any of the PA branches, angioplasty was performed by placing the ductal stent across the stenosis with or without post-dilatation in some patients, or re-crossing a stent strut with a wire and performing balloon angioplasty of the stenotic segment in the others. Procedural success was defined as successful stent deployment with brisk flow into the PA branches and post-procedural SO2 ≥80%. All patients received heparin for 48 hours. Antiplatelet agents were started immediately and continued until definitive surgery.
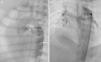
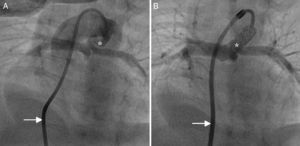
Retrograde ductal stenting in a neonate with tricuspid atresia and pulmonary atresia. (A) Ductal angiogram through a retrograde Judkins right catheter (arrow) showing duct (asterisk) arising from proximal descending aorta; (B) post-stenting ductal angiogram in the same patient showing ductal stent with good flow (asterisk) supplying confluent pulmonary artery branches.
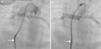
Antegrade ductal stenting in a neonate with pulmonary atresia and ventricular septal defect. (A) Ductal angiogram through a transvenous Judkins right catheter (arrow) showing a tortuous duct (asterisk) arising vertically and proximally from the undersurface of the aortic arch; (B) post-stenting ductal angiogram through a transvenous Mullins sheath and Judkins right catheter (arrow) in the same patient, showing ductal stent with good flow (asterisk).
Patients were followed at one month, three months and three-monthly thereafter, assessing SO2, anthropometry, stent patency and PA branch sizes on echocardiography. MDCT was performed at six months. These parameters were compared with baseline values (Figure 3). Details of re-interventions and definitive surgeries were noted.
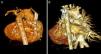
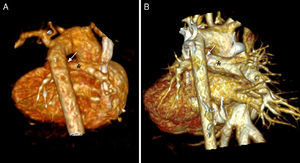
Volume-rendered three-dimensional computed tomography images of an infant with double-outlet right ventricle and pulmonary atresia. (A) Baseline image showing tortuous duct with constrictions (arrow) and confluent pulmonary branches (asterisk); (B) follow-up image after six months showing duct with good ductal flow (arrow) and pulmonary branches with appropriate growth (asterisk).
All variables except age, weight and Z-scores of PA branches followed a Gaussian distribution and were expressed as mean±standard deviation. Medians (range) were provided for age, weight and Z-scores of PA branches. Univariate analysis of continuous data was performed using the t test. The Wilcoxon paired signed-rank test was performed for non-normally distributed variables. Categorical data were analyzed with Fisher's exact test. A p-value <0.05 was considered to indicate significant difference. The subgroup of patients with hypoplasia of any PA branch (Z-score <2.0) was also analyzed separately. The statistical calculations were performed with MedCalc software version 12.2.1.0 (Mariakerke, Belgium).
ResultsBaseline characteristicsSixty patients (41 male), with a median age of 12 (1-1095) days and weight of 2.8 (2.2-8.9) kg, were enrolled. Median right PA (RPA) and left PA (LPA) Z-scores were -1.23 (-10.54 to 2.81) and -0.96 (-8.03 to 3.0), respectively. Three patients older than one year were enrolled for ductal stenting. In these patients, definitive intracardiac repair with insertion of an RV-to-PA conduit was not possible as they had hypoplastic PA branches (Z-scores of one PA branch was -2.77, -10.5 and -6.05, respectively). Two patients with RPA Z-score >2.0 were considered for ductal stenting. One of these was underweight (3.7 kg) and the other weighed 4.01 kg, was just two months old and had hypoplastic LPA. We also enrolled two patients with LPA Z-score >2.0. One had hypoplastic RPA (Z-score -2.77), while the other was just two months old and weighed 2.4 kg. Ductal stenting was performed in these cases to promote balanced PA growth and weight gain. Twenty-five patients had hypoplasia of one of the PA branches. Median RPA and LPA Z-scores were -2.38 (-10.54 to 0.79) and -2.14 (-8.03 to 3.0), respectively, in this subgroup. Among these, eight patients had hypoplasia of both PA branches. In addition, there was PA branch stenosis in nine patients. Twenty-six neonates were prostaglandin-dependent. The duct was arising vertically underneath the arch in 36 patients and was tortuous in 40 patients (Table 1).
Baseline characteristics of patients.
| Age (days), median (range) | 12 (1-1095) |
| Weight (kg), median (range) | 2.8 (2.1-8.9) |
| Gender | |
| Male | 41 |
| Female | 19 |
| Diagnosis | |
| PA-intact ventricular septum | 10 |
| Tetralogy of Fallot-PA | 22 |
| DORV-PA | 13 |
| Tricuspid atresia-PA | 12 |
| CCTGA-PA | 1 |
| DILV-PA | 1 |
| Transitional AVCD-PA | 1 |
| Situs | |
| Solitus | 56 |
| Inversus | 2 |
| Ambiguous | 2 |
| Mechanical ventilation | 8 |
| SO2 (%), mean±SD | 68.88±7.47 |
| Z-score, median (range) | |
| Right pulmonary artery | -1.23 (-10.54 to 2.81) |
| Left pulmonary artery | -0.96 (-8.03 to 3.0) |
| Duct | |
| Narrowest diameter (mm), mean±SD | 1.73±0.57 |
| Length (mm), mean±SD | 12.78±3.32 |
| Origin | |
| Proximal descending aorta | 25 |
| Underneath the arch | 35 |
| Shape | |
| U-shaped curve | 4 |
| Tortuous with multiple loops | 35 |
| Straight tubular | 5 |
| Conical | 16 |
AVCD: atrioventricular canal defect; CCTGA: congenitally corrected transposition of the great arteries; DILV: double inlet left ventricle; DORV: double-outlet right ventricle; PA: pulmonary atresia; SD: standard deviation.
Procedural details were as shown in Table 2. Retrograde arterial access was obtained in 33 patients, via the axillary artery in five patients and via the femoral artery in 28 patients. Stenting was performed via femoral venous access in 27 patients with pulmonary atresia-ventricular septal defect (VSD). In 19 patients, the floppy-tipped wire was replaced with an extra support wire. A total of 64 stents of mean diameter 4.21±0.32 mm and length 14.34±3.44 mm were deployed in ducts of mean narrowest diameter 1.73±0.57 mm and length 12.78±3.32 mm in 59 patients. The procedure was unsuccessful in one patient, as due to extreme proximity and tortuosity of the duct, a wire could not be negotiated. The narrowest ductal diameter increased significantly from a baseline value of 1.73±0.57 mm to 3.67±0.7 mm (p<0.0001). Nine patients had stenosis of one of the PA branches. In four of these, the stent was placed across the stenosis. In another five patients, the stent struts were re-crossed with wires and balloon angioplasties of the stenosis were performed. The procedural success rate was 98.33%. It was 100% with the transvenous route. Post-stenting mean SO2 increased significantly from baseline value of 68.88±7.47% to 90.43±6.04% (p<0.0001). Only two patients suffered complications, pulmonary edema in one and acute stent occlusion in the other. In addition, one patient treated by an antegrade route developed transient atrioventricular conduction disturbances during negotiation of the VSD. Only 14 patients required mechanical ventilation. Patients were discharged after a median hospital stay of four (2-12) days. The complication rate (3.3%) was lower compared to major complications after shunt surgery requiring re-exploration (8.4%) at our institute.
Procedural details.
| Access | |
| Femoral artery | 28 |
| Femoral vein | 27 |
| Axillary artery | 5 |
| Sheath | |
| 4F | 40 |
| 5F | 19 |
| 6F | 1 |
| Mullins sheath | 29 |
| Catheters | |
| JR | 42 |
| IMA | 18 |
| Guidewire negotiated in view | |
| LAO cranial | 28 |
| LAO | 15 |
| LAO caudal | 2 |
| PoA | 5 |
| PoA cranial | 2 |
| RAO cranial | 5 |
| RAO | 2 |
| Guidewire anchored in | |
| MPA | 1 |
| RPA | 26 |
| LPA | 32 |
| Stent | |
| Name | Driver |
| Number | 64 |
| Stent size (mm), mean±SD | 4.21±0.32 |
| Length (mm), mean±SD | 14.34±3.44 |
| Post-dilatation and angioplasty of PA branches | 9 |
| Fluoroscopy time (min:sec), median (range) | 11:54 (3:14-56:12) |
IMA: internal mammary artery catheter; JR: Judkins right coronary catheter, LAO: left anterior oblique; LPA: left pulmonary artery; MPA: main pulmonary artery; PA: pulmonary artery; PoA: posteroanterior; RAO: right anterior oblique; RPA: right pulmonary artery.
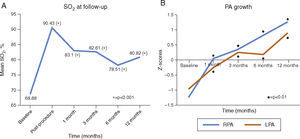
Figure 4 shows mean SO2 and median Z-scores of PA branches at follow-up. At a median follow-up of eight (2-14) months, mean SO2 (80.04±7.54%) was significantly higher than the baseline value of 68.88±7.47% (p<0.0001). Median RPA and LPA Z-scores were 0.56 (-2.89 to 3.29) and -0.02 (-2.81 to 3.86), respectively. The Wilcoxon paired signed-rank test showed that at a median follow-up of eight months, ductal stenting led to a significant increase in PA branch Z-scores (p<0.001). Only four patients had PA branch hypoplasia at follow-up, in contrast to 25 patients prior to ductal stenting (p<0.0001). For this hypoplastic PA subgroup, median RPA and LPA Z-scores were 0.17 (-2.89 to 2.88) and -0.32 (-1.82 to 3.8), respectively, at follow-up. The Wilcoxon paired signed-rank test showed that Z-scores of PA branches at follow-up were also statistically significantly higher than baseline values (p<0.001) in this subgroup. None had hypoplasia of both PA branches at follow-up, in contrast to eight patients initially (p=0.006).
Follow-up data. (A) Arterial oxygen saturation (SO2) at baseline and at different intervals (p-value shows the difference between SO2 at that follow-up and baseline value on t test); (B) Z-scores of pulmonary artery (PA) branches at baseline and at different intervals showing parallel growth of the PA branches at follow-up (p-value shows the difference between Z-scores of the PA branch at that follow-up and baseline value on Wilcoxon paired signed-rank test).
Six patients died, of whom three died due to sepsis: one death (1.6%) was related to ventilator-acquired pneumonia and the others after one month. Another three died of worsened cyanosis due to impaired PBF, probably due to ductal occlusion, one at one month and the others between two and three months. In the first patient death was likely caused by stent thrombosis (ST) due to dehydration related to diarrhea, and re-intervention was not possible as the patient died immediately at hospital admission. Another two patients died at remote hospitals or in transit after a brief period of worsened cyanosis and hypoxia noticed by primary care physicians. At our institute, during the study period, 210 patients underwent aortopulmonary shunt procedures, of whom 33 (15.7%) suffered in-hospital mortality. In-hospital mortality with ductal stenting (1.6%) was significantly lower than that (p=0.0018).
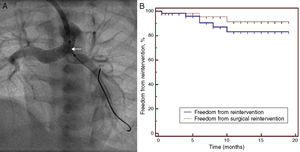
Re-interventionSix patients required re-intervention. Of these, only three required surgical re-intervention in the form of aortopulmonary shunt. One patient required aortopulmonary shunt immediately post-procedure and another two after six months as they were underweight for definitive surgery. Three patients were successfully managed with catheter-directed re-interventions only. One patient required repeat stenting for restenosis at the aortic end at four months and another two required repeat angioplasty for stenosis of the PA branches (stenting in one at eight months and balloon angioplasty in the other at six months) to promote proper balanced growth of PA branches prior to surgery. At six months, freedom from re-intervention was 90.5% and that from surgical re-intervention was 95.4% (Figure 5).
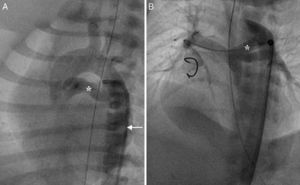
Re-interventions. (A) Re-intervention via an axillary artery route for left pulmonary artery (LPA) stenosis showing negotiation of a balloon (arrow) through the ductal stent into the LPA; (B) Kaplan-Meier analysis showing freedom from re-intervention and from surgical re-intervention at follow-up.
At a median follow-up of eight (5-11) months, definitive surgeries were performed in 35 patients (biventricular repair in 19 and Glenn shunt in 16 patients), with successful removal of the stents.
DiscussionInfants with duct-dependent PBF require early palliation. Although prostaglandin E1 provides an immediate pharmacological palliation, prolonged infusion causes peripheral vasodilation, hypotension, hyperpyrexia, enteropathy and apnea.7 Subsequent shunting of blood from the systemic to the pulmonary circulation can be accomplished by either the well-established surgical systemic-to-pulmonary shunt procedure1,8 or newer percutaneous approaches.9,10 Systemic-to-pulmonary shunts are associated with significant complications in younger children, including hemorrhage, poor flow, blockage/narrowing or thrombosis of the shunt, over-shunting, infection, seroma, chylothorax, phrenic nerve injury leading to diaphragmatic palsy, vagal nerve injury, vocal palsy, limb compromise due to subclavian artery damage, infective pulmonary endarteritis, and pleural collections.11–15 At our institute 8.4% of patients required re-exploration after shunt surgery. Mortality after neonatal modified Blalock-Taussig shunt (MBTS) remains high (7.2-11%), particularly in infants weighing <3.0 kg and aged <30 days.4,16,17 Approximately 33% of early deaths occur within 24 hours and 75% occur within 30 days postoperatively. In addition, 11-13% experience perioperative morbidity.4,18 Overall mortality with MBTS varies between 2.3 and 16%, and infant mortality is 8% in the early post-operative period.17,19–21 In-hospital mortality with shunting at our center is 15.7%, while early (in-hospital) mortality with ductal stenting was 1.6% in our study. In a study comparing MBTS and ductal stenting, initial post-procedural SO2, 30-day survival, overall survival to second-stage palliation, definitive repair, and survival at 12 months were not significantly different between patients receiving MBTS and ductal stenting.22 Progressive endovascular growth with obstruction, distortion, stenosis and differential growth of PA branches, and adhesions may complicate future surgeries.1,3,5,14,18,22 Ductal stenting, a less invasive alternative, obviates many of these complications.15,22,23 It maintains PBF and serves as a bridge toward later repair.5
Difficulties encountered previously during ductal stenting included inability to enter the duct, ductal spasm, and incomplete stenting of full ductal length. Hence, repeat procedures were often required. Duration of palliation was poor due to intrastent endothelial proliferation, especially after six months.11,13,24 With recent-generation delivery systems and stents with better profile, flexibility and trackability, ductal stenting may be achieved easily and safely.6,11,12 Definitive repair at an earlier age and lower body weight and availability of re-dilatation make poor duration of palliation an unimportant issue.6,11
With the arterial route, problems are due to small vessel size.3,6 Exclusion of patients weighing <2.5 kg has been recommended as a 4F sheath may cause major damage to the femoral artery.6 However, 16 of our patients weighed <2.5 kg. Arterial or venous access with 4F sheaths was obtained in all of these, with no vascular damage. The advantage of the arterial route is avoidance of intracardiac catheterization and hence avoidance of associated complications such as arrhythmias. We found the femoral arterial route feasible when the duct was arising from the distal arch or proximal descending thoracic aorta; this kind of ductal morphology was found most commonly with pulmonary atresia-intact ventricular septum (IVS) and tricuspid atresia, and in these patients we performed ductal stenting via a femoral arterial route.
The duct in some lesions may be long and tortuous, with one or more curves and constrictions. It sometimes arises proximally and vertically from the aorta (especially in pulmonary atresia-VSD and univentricular physiology) and may have a kink at the pulmonary end where it inserts into a PA with a constriction.3,6,11 Difficulty in engaging the ductal ampulla and securing a stable wire position for advancing a stent-mounted balloon catheter through the duct has been reported with the femoral arterial route.11 This is because it allows only a short length of a guidewire to be passed into the PA. Spasm and complete obliteration of the duct during attempts to cross it with an over-the-wire catheter have also been reported. This is true especially in patients with pulmonary atresia-VSD with a vertical and tortuous duct with severe narrowing.3,6,25 Stenting has also been performed transvenously through the pulmonary valve after transcatheter relief of RV outflow obstruction.12,15,25 However, in contrast to pulmonary atresia-IVS, atresia in pulmonary atresia-VSD involves long segments, making transcatheter relief of obstruction impossible. Access to the duct transvenously through a VSD has been reported.11,26,27 In 27 patients with pulmonary atresia-VSD, we performed ductal stenting through the femoral venous route. A catheter was negotiated via the RA, RV and through the VSD into the aorta to approach the duct. The limitations of the transvenous route are need for intracardiac catheterization and negotiation of the VSD.
Ductal stenting has also been attempted via axillary or carotid artery cannulation or cut-down.5,15,26,28 In patients with a proximal duct, we performed ductal stenting via the axillary artery in five patients at primary intervention and in one patient at re-intervention. However, all these routes suffer limitations related to small artery size and access site complications.
ProcedureProstaglandin infusion was stopped several hours before the procedure to have a reasonably constricted duct, as a large duct (≥2.5 mm) is not suitable for stenting due to the risk of stent migration.5,6,11,25 Meticulous investigation of ductal and PA anatomy is essential on initial angiography.5,6,11 Lateral and 4-chamber (left anterior oblique [LAO] view with cranial angulation) views have been recommended to open up a PA bifurcation and to show proximal LPA stenosis if present.6,11 The duct was negotiated in LAO cranial view in 43 of our patients. A JR or IMA catheter was passed from the femoral vein, femoral artery or axillary artery to the aorta. With the femoral venous route, extra back-up coronary guiding catheters, with or without the tip cut off to form an inverted ‘J’, and Judkins right or left guiding catheters have been used to engage the ampulla of the duct arising vertically from the undersurface of the aortic arch in some studies.6,11,27 A stable guidewire position is easily achieved from the venous route.3 A wire with a short, floppy hydrophilic tip for negotiating a long and tortuous duct with constriction and a relatively stiff body to enable tracking of the balloon stent across the duct is recommended.3,6 Some operators have used a microcatheter to exchange the soft guidewire for a stiffer one to ease stent delivery.5 We advanced the stiffer wire through the duct straightened with a floppy-tipped wire in situ to exchange with an extra-support wire in 19 of our patients. Then the latter was removed and a stent could be tracked over the stiffer wire, as reported previously.6 Sufficient length of wire should be anchored securely in a distal PA branch.6 The guiding catheter and Mullins sheath provided better support to track the balloons and stents through the tortuous ducts in our study.
StentsA pre-mounted BMS with open-cell design is preferred.5,6 Peripheral stents have also been used in older children.26 We used cobalt-chromium stents with an open-cell design to ease delivery through the tortuous ducts. Stent length was selected to cover the duct completely and slightly longer than duct length. Measuring tortuous and angled ducts accurately was difficult. Therefore, we measured ductal length only after the duct was negotiated and straightened with a wire, as advocated in some studies.5,6,11 We tried to ensure that the stent covered the duct completely, especially at the pulmonary end. It should be slightly (1-2 mm) longer than the duct, expecting 10-20% foreshortening with full expansion. This is to prevent spasm as unstented segments have a propensity for constriction.3,6,11,12,15,25,29 We performed repeat angiograms using small contrast volume with hand injections through the guide catheter or Mullins sheath as needed to facilitate correct stent placement. Fine adjustments were performed to position the stent accurately, so that there was sufficient stent length in the main PA and no protrusion into the aorta at the other end, as it has been shown that a stent protruding into the aorta may be difficult to re-cross at follow-up catheterization.6
In contrast to MBTS, a stented duct is more comparable with a central shunt, with no restriction at the aortic end.26 We selected stent diameter so as to provide adequate PBF with relief of constriction. A 4.0 mm diameter stent for patients weighing 3.0-4.0 kg, a 4.5 mm diameter stent for those weighing 4.0-5.0 kg and a 3.5 mm diameter stent for patients weighing <3.0 kg is recommended.6,11 Predilatation of a tightly stenotic duct followed by measurement of ductal diameter on the angiogram has been advised.26 We selected the stent diameter keeping the largest diameter (most commonly at the ampulla near the aortic end) as a reference. Stents were deployed at high pressures and post-dilatation was performed if required. A final post-stenting angiogram with a guidewire in situ was performed in all patients to confirm patency of the duct and PA branches. This was to ensure that the delivery of another stent was feasible if required, especially in the rare case of acute ST.6
ComplicationsPrevious studies reported low periprocedural complication rates: acute ST (2-3%), ductal spasm (less than 1%), stent dislodgement and migration, vessel or chamber damage or perforation, pulmonary hemorrhage, and others.5,6,11,23,29–31 The media of the ductus arteriosus is composed primarily of muscle fibers and its intima is thicker than other vessels. These properties may cause fatal ductal spasm.32 None of our patients suffered access-related damage, spasm, stent migration or incomplete stenting. Acute stent occlusion probably due to ST occurred in one patient and was managed with aortopulmonary shunt. One patient suffered pulmonary overflow. With the transvenous route, heart block can occur as a stiff guiding catheter or long sheath rub against a VSD rim where a conduction bundle is located.6 Only one of our patients suffered such atrioventricular conduction disturbances and these were only transient. Very few immediate procedure-related deaths have been reported.30,31
Long-term complications resemble those of surgical shunts and consist of progressive stent stenosis, overflow, pulmonary hypertension and distortion of PA branches.11 Intimal proliferation, contraction of the vessel wall and slowly progressive stent stenosis are major limitations.11,12,15,32 In such patients, we may re-dilate and/or implant an another stent,11,15 as occurred in three of our patients.
Pulmonary artery branch stenosisStenting provokes intense neointimal proliferation and fibrosis in ductal tissues that extends into the media of the PA wall. It tends to accelerate pre-existing PA branch stenosis, requiring a salvage shunt.3,6,11,23 This is especially true for patients with pulmonary atresia-VSD with a tendency for ductal insertion into the proximal LPA.6 Avoidance of ductal stenting was therefore recommended in such patients, especially for those who will undergo univentricular repair.6,11,33 However, stenting of PA branches or bifurcation stenting of the PA confluence simultaneously with ductal stenting is reported recently.26,28 We performed dilatation with or without stenting of PA branches in nine patients during the primary procedure and in two patients during re-intervention. The number of patients with hypoplasia of one or both PA branches at follow-up was significantly less than that at the beginning of our study.
Follow-upIn our study, ductal stenting provided adequate SO2 immediately and at follow-up. In previous studies, SO2 was maintained at follow-up (mean of 82% at median follow-up of 13 months in one study26) and was comparable to that following a surgical shunt.30 Echocardiography and/or angiocardiography were used to assess PA anatomy at follow-up.11,26,28,34 We used echocardiography and MDCT.
Aortopulmonary shunt and ductal stenting both promote a significant increase in the Nakata index and McGoon ratio.30,34 However, an MBTS may worsen the left-to-right PA diameter ratio, due to preferential growth of the PA branch contralateral to a shunt. In contrast to a shunt, PA narrowing remote from the ductal insertion site was not observed in any of the ductal stenting patients in our study, similarly to a previous study.22 However, since the stent is implanted in a natural position of the duct, it results in a better angle between the duct and PA branches and promotes more evenly distributed PBF and thereby more uniform PA development, without causing distortion or stenosis,15,30,34 as well as enabling future definitive repair.26 Our study showed balanced growth of PA branches. Mortality in three cases with worsened cyanosis and hypoxia was probably due to sudden impairment of PBF due to ductal occlusion.
Re-interventionIn most studies, early re-intervention consisted of re-stenting to overcome constriction of the unstented ductal segments.5 Some have reported no restenosis at six months.30 Intimal proliferation leading to progressive in-stent stenosis and reduced SO2 with need for balloon angioplasty, re-stenting or surgery by six months have also been reported.5,11,15,26 In one study, rates of re-interventions to maintain adequate PBF were not significantly different between those who received MBTS and those treated by ductal stenting.22 However, the median interval to re-intervention was shorter with MBTS than that with ductal stenting; there was a need for multiple percutaneous re-interventions for contralateral PA stenosis in the MBTS group, while no patient in the ductal stenting group required an intervention for distal, non-juxtaductal narrowing. In addition, in contrast to the MBTS group, no patient in the ductal stenting group required more than one re-intervention.22 We succeeded in performing catheter-directed re-interventions in three out of six patients with restenosis, with 95.4% freedom from surgical re-interventions at six months.
Definitive surgeryThe durability of palliation by ductal stenting is generally less compared to that from a surgical shunt; hence, close follow-up and definitive surgery within 6-18 months are advocated.6,11 At surgery, we found reasonable PA growth, as reported in most studies.5,11–13,15,23,29–31 Recently, most studies like ours have found that most stents were easily removed, with very few patients requiring reconstruction for PA distortion.
ConclusionArterial duct stenting overcomes the limitations of shunt surgery and thoracotomy in patients with duct-dependent pulmonary circulation. It maintains adequate SO2 and promotes balanced PA growth at intermediate follow-up. It provides reasonable mid-term palliation in these patients until they can receive definitive repair.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.