Calcific aortic stenosis (CAS) is a clinical entity with an increasing prevalence in developed countries as populations age.1 The prevalence of CAS and its initial stage, aortic sclerosis, is reaching epidemic proportions, affecting 2-7% of the general population and 30% of adults aged over 65.2
After becoming symptomatic, CAS patients have an increased risk of sudden death and a mean survival of 2-3 years,1 and so prompt aortic valve replacement (AVR) is recommended. AVR is currently the only definitive treatment available with proven benefits in symptom relief and improved survival.1 However, the diagnostic criteria for CAS and the optimal timing for AVR are the subject of debate, mainly because symptom severity is often misjudged or masked by aging and the presence of other comorbidities. Studies have consistently shown that mortality in CAS patients awaiting surgery is higher than in those referred for isolated coronary surgery.3 Additionally, even after successful valve replacement, at least 15% of patients die within one year and another 20% experience a serious event. Therefore, there is a need for biomarkers that can monitor the severity, progression and prognosis of CAS, since they could improve risk stratification and clinical decisions, particularly with regard to the optimal timing for AVR, since plasma markers can be accurately measured in the laboratory to give robust, cost-effective and rapid results. However, the value of the available markers is debatable, and none have yet been translated into clinical practice or incorporated into the guidelines.
The pathophysiology of CAS involves various biological processes and has not been completely elucidated. Briefly, CAS is triggered by mechanical stress on the aortic valve in conjunction with atherosclerotic risk factors, leading to endothelial dysfunction and valve leakage, followed by deposition and oxidation of lipids and other compounds in the subendothelium. Monocytes infiltrate the valve tissue and phagocytize the modified lipids, becoming foam cells. T lymphocytes secrete cytokines, which promote inflammation and remodeling of the extracellular matrix. Fibroblasts transdifferentiate into valvular myofibroblasts with an osteoblast-like phenotype. These events underlie subsequent changes involving extracellular matrix remodeling and neovascularization, ultimately leading to active calcification.4 Given the multiple biological pathways underlying the pathophysiology of CAS, there are many potential biomarkers that could be clinically valuable to diagnose CAS and monitor its progression and prognosis.
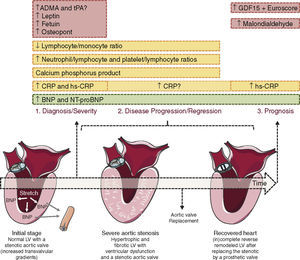
Most of the markers studied so far have been used to assess the presence and severity of CAS but are less useful for monitoring progression or prognosis (Figure 1). An exception seems to be B-type natriuretic peptide (BNP) and its prohormone, NT-proBNP, released by cardiomyocytes on stretching. Increased BNP levels are associated with low-flow CAS, impaired left ventricular (LV) longitudinal strain and myocardial fibrosis (Figure 1). BNP appears to be an independent predictor of prognosis in patients with CAS, particularly in those with severe stenosis and low transvalvular gradient. In CAS, patients with higher BNP or NT-proBNP levels display significantly lower one-year survival and higher probability of developing symptoms, respectively.5
Biomarkers of aortic valve stenosis. Ideally a biomarker should be easily measurable, highly reproducible and useful in diagnosing (1), following the disease progression (2) and predicting its prognosis (3). Most of the markers studied so far are valuable tools to diagnose and assess the severity of aortic valve stenosis (AVS, ex. CRP and osteopontin) but show poor utility when it comes to monitor AVS progression or prognosis. The exception seems to be B-type natriuretic peptide (BNP) and its prohormone, NT-proBNP, both able to diagnose, assess the severity and prognosis of AVS patients. BNP mechanism of release is triggered by ventricular pressure overload imposed by the stenotic valve as depicted in the heart on the left. ADMA, asymmetric dimethylarginine; CRP, C-reactive protein; GDF-15, growth differentiation factor-15; hs-CRP, high-sensitivity; tPA, enzyme tissue plasminogen activator. Figure was produced using Servier Medical Art.
Markers of endothelial dysfunction such as asymmetric dimethylarginine (ADMA), enzyme tissue plasminogen activator (tPA) and homocysteine have shown modest results. While high plasma levels of both ADMA and tPA are independently linked to a diagnosis of CAS, the latter was found not to be a predictor of CAS.4,6 Subsequent studies with smaller number of patients have shown contradictory results, highlighting the weaknesses of these biomarkers.
Markers of oxidative stress, such as malondialdehyde, 8-hydroxy-2-deoxyguanosine, cysteine, homocysteine, cysteinylglycine and glutathione, have also been evaluated. Among these, only malondialdehyde has proved promising, predicting adverse outcomes during 30-day and 1-year follow-up in high-risk patients with symptomatic, severe CAS treated with transcatheter AVR.7
Hypercholesterolemia has also been associated with the pathogenesis of CAS, despite contradictory results from studies assessing the use of lipid-lowering agents in these patients. Thus research on potential CAS markers related to lipid deposition, such as total, LDL and oxidized-LDL cholesterol plasma levels became promising. Disappointingly, several major studies (SEAS, SALTIRE and ASTRONOMER8–10) have shown no direct relationship between the progression of CAS and any cholesterol-related particles. Similarly, leptin levels have been significantly associated with the presence of CAS, although the role of leptin in its pathogenesis and whether it is associated with the progression of CAS remains unclear.4
Another potential group of biomarkers is molecules involved in osteoblastic transdifferentiation, such as fetuin, which inhibits calcification and whose plasma levels are inversely associated with the presence of CAS, but only in non-diabetic patients. The relation of fetuin levels with the severity and progression of CAS is unclear.11 Osteopontin is another potentially interesting marker for CAS, since it is the only molecule directly involved in the ectopic calcification that occurs in the later stages of the disease. Recently a correlation between plasma osteopontin levels and the severity of CAS has been demonstrated.4 Lastly, calcium-phosphorus product is associated with the severity of CAS in patients with end-stage renal disease, in which it is inversely related to aortic valve area and positively related to both peak and mean transvalvular gradients.4
Over the last decade, the concept of CAS as a degenerative disease has changed due to increasing evidence of an active inflammatory process related in many ways to arteriosclerosis,11 prompting exploration of inflammatory molecules as potential biomarkers of CAS progression. Interventions that reduce the degree of inflammation have been shown to attenuate the progression of valve stenosis.12 In this regard, C-reactive protein (CRP) and high-sensitivity CRP plasma levels have been shown to be elevated in CAS, although with no association with aortic valve area, degree of calcification or aortic jet velocity, and to discriminate patients with and without events, respectively. Regarding disease progression and prognosis, the usefulness of CRP levels is uncertain, mostly due to its non-specific mode of release during most inflammatory processes.4 Recently, combining growth differentiation factor-15 levels with the logistic Euro-SCORE or EuroSCORE II has led to the identification of patient subgroups with greatly differing outcomes after transcatheter AVR.13 The neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio have also recently been the subject of interest as predictors of the severity and extent of CAS.14,15 Both ratios significantly correlated with the severity of CAS and the latter also predicted the presence of LV systolic dysfunction.
Another promising disease marker is the lymphocyte/monocyte ratio. In the current issue of the Journal, Efe at al.16 observed a statistically significant inverse relationship between this ratio and severity of CAS. Despite its inherent limitations this study makes an additional and promising contribution to this exciting emerging field and paves the way for future research in this topic.
It is worth mentioning other potential CAS markers that have been assessed with modest results. Indexes of von Willebrand factor activity have been associated with CAS severity and bleeding and were predictive of cardiovascular outcomes17; extracellular matrix proteins, such as MMP-1, MMP-2, MMP-9 and TIMP-1, displayed similar values between CAS patients and controls despite showing univariate correlations with echocardiographic data (LV enlargement and diastolic function); and inflammatory biomarkers, including interleukin-1 beta, tumor necrosis factor alpha and transforming growth factor beta, correlated with fibrotic markers in patients with mild CAS but were unable to discriminate CAS.18
As a consequence of rising life expectancy, the demand for aortic valve monitoring can be expected to increase in the future. The initial stages of CAS usually remain asymptomatic for a long time; when patients complain the disease has already progressed to an advanced stage. The more information is gathered on the natural course of aortic valve degeneration, the greater the chances of successfully intervening before irreversible valve damage has ensued and surgery becomes urgent. Therefore, instead of using a single plasma marker, there are high hopes for the potential utility of a panel of biomarkers, reflecting diverse pathways involved in CAS, or even a scoring system that combines biomarkers with echocardiographic and/or clinical data to aid in risk stratification of patients with CAS.
Conflicts of interestThe authors have no conflicts of interest to declare.