Calcific aortic valve disease, a chronic progressive disorder, is the leading cause of valve replacement among elderly patients. The lymphocyte/monocyte ratio has been recently put forward as an inflammatory marker of relevance in several cancers as well as in cardiovascular disease. This study aims to assess the correlation between severity of calcific aortic stenosis and the lymphocyte/monocyte ratio.
MethodsThe study retrospectively included 178 patients with a diagnosis of calcific aortic stenosis and 139 age- and gender-matched controls. The patients were divided into two groups according to the severity of aortic stenosis: mild-to-moderate and severe.
ResultsAn inverse correlation was discerned between the severity of the aortic stenosis process (mean gradient) and the lymphocyte/monocyte ratio (r=-0.232, p=0.002). The lymphocyte/monocyte ratio was observed to decrease as the severity of aortic stenosis increased (p<0.001) in the group with severe aortic stenosis compared with the mild-to-moderate aortic stenosis and control groups (p<0.001, p=0.005 respectively), and in the group with mild-to-moderate aortic stenosis compared with the control group (p=0.003). Multivariate regression analysis revealed that the lymphocyte/monocyte ratio was independently related to the severity of calcific aortic stenosis (p=0.003).
ConclusionThe present study demonstrated the existence of a statistically significant inverse relationship between severity of calcific aortic stenosis and the lymphocyte/monocyte ratio. The study also revealed that the lymphocyte/monocyte ratio was significantly related to the severity of the aortic valve stenosis process.
A doença valvular aórtica calcificada, perturbação progressiva crónica, é a principal causa de substituição de válvulas nos doentes idosos. A relação linfócito/monócito tem sido recentemente apresentada como um marcador inflamatório de relevância no caso de diversas neoplasias, bem como de doenças cardiovasculares. Este estudo visa avaliar a correlação entre a gravidade da estenose aórtica calcificada e a relação linfócito/monócito.
MétodosO estudo retrospetivo incluiu 178 doentes com um diagnóstico de estenose aórtica calcificada e 139 controlos emparelhados para a idade e o género. Os doentes foram divididos em dois grupos de acordo com a gravidade da estenose aórtica: (a) suave-moderada e (b) grave.
ResultadosUma correlação inversa foi identificada entre a gravidade do processo da estenose aórtica (gradiente médio) e a razão linfócito/monócito (r = -0,232, p = 0,002). Observou-se que a razão linfócito/monócito diminuía à medida que a severidade da estenose aórtica aumentava (p <0,001) no grupo com estenose aórtica grave, quando comparado com o grupo com a estenose aórtica ligeira-moderada e com os grupos controlo (p <0,001; p = 0,005 respetivamente), bem como no grupo com estenose aórtica ligeira-moderada, quando comparados com o grupo controlo (p = 0,003). A análise da regressão multivariada revelou que a relação linfócito/monócito está relacionada independentemente da gravidade da estenose aórtica calcificada (p = 0,003).
ConclusãoO presente estudo demonstrou a existência de uma relação estatística significativamente inversa entre a gravidade da estenose aórtica calcificada e a relação linfócito/monócito. O estudo também revelou que a relação linfócito/monócito estava significativamente relacionada com a gravidade do processo da estenose valvular aórtica.
Calcific aortic stenosis (CAS), an ever-increasing public health problem among elderly patients, is the leading cause of valve replacement within this age group.1,2 The pathological process of the disease involves aortic valve thickening (sclerosis) along with fibrosis and calcification. Inflammation plays an important role in the development and progression of both aortic sclerosis and calcification.3,4 This is reinforced by the observation that interventions that decrease exposure to inflammation have the potential to alleviate progressive stenosis in the aortic valve.4 Therefore, the identification of inflammatory markers implicated in CAS can potentially be helpful in assessing the progression as well as the severity of the disease. A previous study reported the relationship between severity of CAS and the neutrophil/lymphocyte ratio (NLR), which is an inflammatory marker calculated from blood count parameters.5
Several studies have shown that the lymphocyte/monocyte ratio (LMR) is a convenient and useful marker for systemic inflammation in different malignancies and that it is closely associated with disease prognosis.6–8 A recent study reported that LMR is related to in-stent restenosis and another study proposed that LMR could serve as an indicator for mortality in heart failure.9,10 This study is aimed at investigating the relationship between the severity of aortic stenosis and LMR in the specific case of CAS, which is a disease closely associated with inflammation.
MethodsThis study retrospectively included 178 patients diagnosed with CAS between April 2012 and January 2016 along with 139 age- and gender-matched controls. The control group were chosen from cardiology outpatients without prior cardiovascular disease history who were admitted for a general check-up or with atypical and/or non-cardiac complaints. Among these, individuals who underwent treadmill exercise tests and/or myocardial perfusion scintigraphy with negative test results were recruited as controls. Patients with CAS were investigated for mean aortic gradient as assessed by transthoracic echocardiography and the results were used to divide the patient pool into two groups according to disease severity: mild-to-moderate (111 patients) and severe (67 patients).
The exclusion criteria for both patient and control groups were: presence of congenital or rheumatic aortic valve disease; pre-existing diagnosis or suspicion of coronary artery disease (positive stress tests for coronary artery disease and/or any chest pain considered to be angina); left ventricular dysfunction; atrial fibrillation; hemodynamically significant arrhythmia; severe stenosis or regurgitation in other heart valves; active or chronic infection; systemic inflammatory or allergic disease; and presence of renal, hepatic or hematologic disease. Patients whose clinical, laboratory or echocardiographic data were not available on medical databases used in the study were also excluded.
Patients’ clinical and demographic data and laboratory results relevant to the study were obtained from the hospital information management system as well as patient files. Routine biochemical blood tests, lipid panel and complete blood counts were evaluated for the purpose of this study. The control group of the study consisted of 139 age- and gender-matched individuals who were confirmed not to have significant valve disease and/or cardiac dysfunction following echocardiographic evaluation. The patients were evaluated in terms of age, gender, smoking status, and presence of hyperlipidemia or diabetes mellitus. The medications used by the patients were assessed. The LMR was calculated by dividing the number of lymphocytes by the number of monocytes in the peripheral blood count and the NLR by dividing the number of neutrophils by the number of lymphocytes. The study was conducted following approval by the hospital ethics committee.
Echocardiographic evaluation was performed using a Philips iE33 ultrasound system (Andover, MA, USA) and a 2.5-5 MHz transducer. Parasternal long- and short-axis and apical views were used for imaging and taking measurements required for the study. Aortic jet velocity was estimated using Doppler echocardiography. Mild aortic stenosis was defined as mean transaortic pressure gradient less than 25 mmHg or aortic jet velocity between 2.0 and 3.0 m/s, moderate aortic stenosis as a gradient between 25 and 40 mmHg or aortic jet velocity between 3.0 and 4.0 m/s, and severe aortic stenosis as a gradient greater than 40 mmHg or aortic jet velocity greater than 4.0 m/s. All echocardiographic evaluations were performed by an experienced cardiologist.
Statistical analysisStatistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as medians and standard deviation, and categorical variables as percentages. The normal distribution of the data was tested with the Kolmogorov-Smirnov test. For comparison of categorical variables, Pearson's chi-square and Fisher's exact tests were used as appropriate. When two groups were present, the Student's t test was used to compare data with normal distribution, while the Mann-Whitney U test was used to compare data with non-normal distribution. When three groups were present, a one-way ANOVA test was used to compare variables with normal distribution and Tukey's test was performed for post-hoc analysis; the Kruskal-Wallis test was used for comparison of variables without a normal distribution. Linear regression analysis was performed to determine the relationship between severity of CAS and the variables under study. Variables with a p-value of less than 0.1 in univariate linear regression analysis were subsequently used for multivariate linear regression analysis. A p-value of less than 0.05 was considered as significant for multivariate linear regression analysis as well as other tests.
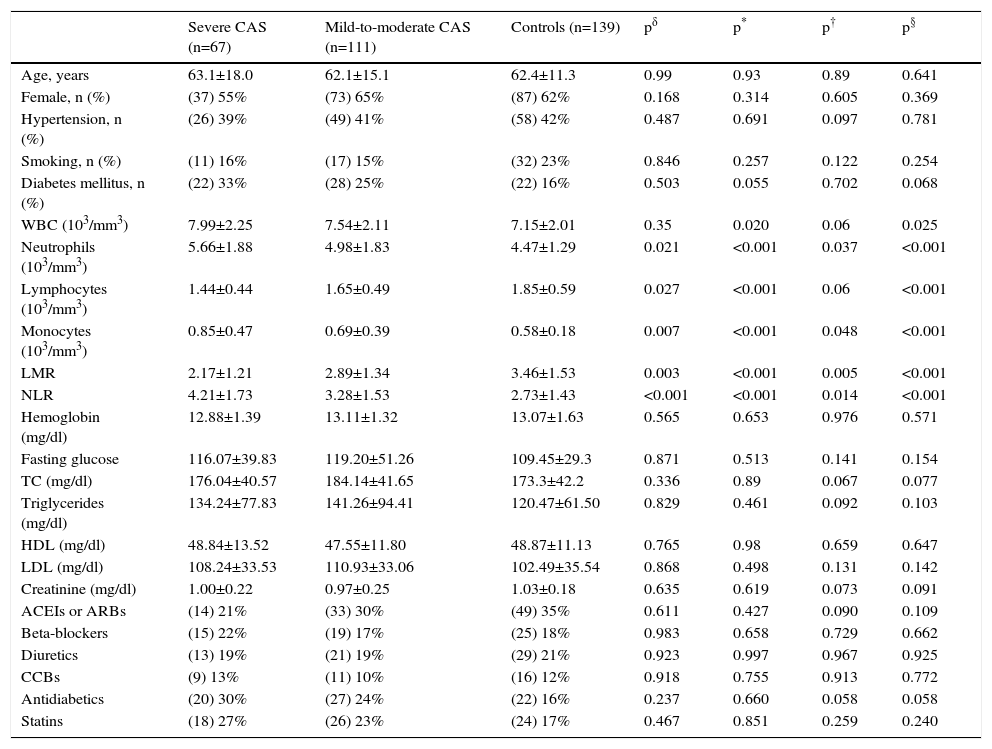
ResultsThe study, which comprised 345317 participants in total, included 67 patients with severe CAS, 111 with mild-to-moderate CAS, and 139 without CAS as control subjects. The patient characteristics and laboratory findings pertinent to the study are summarized in Table 1. There were no notable differences between the groups in terms of age, gender, presence of diabetes mellitus, presence of hypertension, or smoking status (p>0.05 for each). Total white blood cell count and neutrophil, lymphocyte, and monocyte counts were significantly different between groups (p=0.025, p<0.001, p<0.001, and p<0.001, respectively). In the group with severe aortic stenosis, the NLR was significantly higher and the LMR was significantly lower compared to the two other groups (p<0.001 for both). In the group with mild-to-moderate aortic stenosis, the NLR was significantly higher and the LMR was significantly lower in comparison to the control group (p=0.005 and p=0.014, respectively). Lipid profile parameters, creatinine, and hemoglobin values were comparable between the three groups (p>0.05 for each). There were no differences in terms of the use of angiotensin-converting enzyme or angiotensin II receptor blockers, beta-blockers, diuretics, calcium channel blockers, antidiabetics and statins (p>0.05 for each).
Clinical characteristics of patients with calcific aortic stenosis and control subjects.
| Severe CAS (n=67) | Mild-to-moderate CAS (n=111) | Controls (n=139) | pδ | p* | p† | p§ | |
|---|---|---|---|---|---|---|---|
| Age, years | 63.1±18.0 | 62.1±15.1 | 62.4±11.3 | 0.99 | 0.93 | 0.89 | 0.641 |
| Female, n (%) | (37) 55% | (73) 65% | (87) 62% | 0.168 | 0.314 | 0.605 | 0.369 |
| Hypertension, n (%) | (26) 39% | (49) 41% | (58) 42% | 0.487 | 0.691 | 0.097 | 0.781 |
| Smoking, n (%) | (11) 16% | (17) 15% | (32) 23% | 0.846 | 0.257 | 0.122 | 0.254 |
| Diabetes mellitus, n (%) | (22) 33% | (28) 25% | (22) 16% | 0.503 | 0.055 | 0.702 | 0.068 |
| WBC (103/mm3) | 7.99±2.25 | 7.54±2.11 | 7.15±2.01 | 0.35 | 0.020 | 0.06 | 0.025 |
| Neutrophils (103/mm3) | 5.66±1.88 | 4.98±1.83 | 4.47±1.29 | 0.021 | <0.001 | 0.037 | <0.001 |
| Lymphocytes (103/mm3) | 1.44±0.44 | 1.65±0.49 | 1.85±0.59 | 0.027 | <0.001 | 0.06 | <0.001 |
| Monocytes (103/mm3) | 0.85±0.47 | 0.69±0.39 | 0.58±0.18 | 0.007 | <0.001 | 0.048 | <0.001 |
| LMR | 2.17±1.21 | 2.89±1.34 | 3.46±1.53 | 0.003 | <0.001 | 0.005 | <0.001 |
| NLR | 4.21±1.73 | 3.28±1.53 | 2.73±1.43 | <0.001 | <0.001 | 0.014 | <0.001 |
| Hemoglobin (mg/dl) | 12.88±1.39 | 13.11±1.32 | 13.07±1.63 | 0.565 | 0.653 | 0.976 | 0.571 |
| Fasting glucose | 116.07±39.83 | 119.20±51.26 | 109.45±29.3 | 0.871 | 0.513 | 0.141 | 0.154 |
| TC (mg/dl) | 176.04±40.57 | 184.14±41.65 | 173.3±42.2 | 0.336 | 0.89 | 0.067 | 0.077 |
| Triglycerides (mg/dl) | 134.24±77.83 | 141.26±94.41 | 120.47±61.50 | 0.829 | 0.461 | 0.092 | 0.103 |
| HDL (mg/dl) | 48.84±13.52 | 47.55±11.80 | 48.87±11.13 | 0.765 | 0.98 | 0.659 | 0.647 |
| LDL (mg/dl) | 108.24±33.53 | 110.93±33.06 | 102.49±35.54 | 0.868 | 0.498 | 0.131 | 0.142 |
| Creatinine (mg/dl) | 1.00±0.22 | 0.97±0.25 | 1.03±0.18 | 0.635 | 0.619 | 0.073 | 0.091 |
| ACEIs or ARBs | (14) 21% | (33) 30% | (49) 35% | 0.611 | 0.427 | 0.090 | 0.109 |
| Beta-blockers | (15) 22% | (19) 17% | (25) 18% | 0.983 | 0.658 | 0.729 | 0.662 |
| Diuretics | (13) 19% | (21) 19% | (29) 21% | 0.923 | 0.997 | 0.967 | 0.925 |
| CCBs | (9) 13% | (11) 10% | (16) 12% | 0.918 | 0.755 | 0.913 | 0.772 |
| Antidiabetics | (20) 30% | (27) 24% | (22) 16% | 0.237 | 0.660 | 0.058 | 0.058 |
| Statins | (18) 27% | (26) 23% | (24) 17% | 0.467 | 0.851 | 0.259 | 0.240 |
ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; CAS: calcific aortic stenosis; CCBs: calcium channel blockers; HDL: high-density lipoprotein cholesterol; LDL: low-density lipoprotein cholesterol; LMR: lymphocyte/monocyte ratio; NLR: neutrophil/lymphocyte ratio; TC: total cholesterol; WBC: white blood cell count.
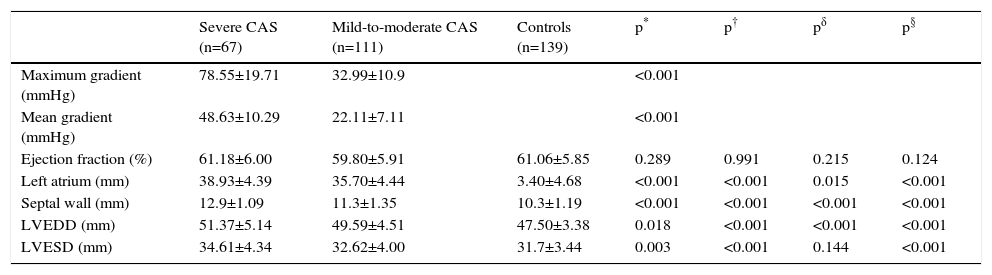
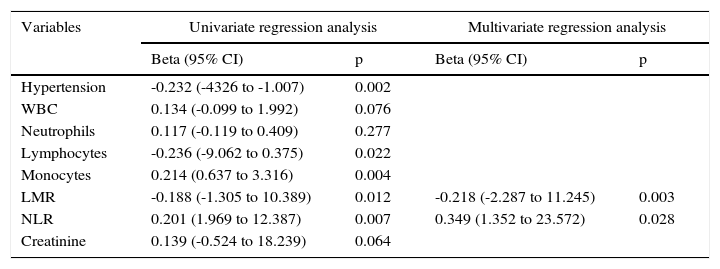
Echocardiographic findings are summarized in Table 2. As expected, mean aortic pressure gradient was higher in patients with severe CAS than in those with mild-to-moderate aortic stenosis. There was no significant difference observed between the three groups with regard to ejection fraction (p>0.05); however, left ventricular end-diastolic diameter, left ventricular end-systolic diameter (LVESD), and left atrial wall and interventricular septal thicknesses were different in each of the three groups. In terms of left atrial diameter and LVESD, post-hoc analysis revealed no significant differences between patients with severe aortic stenosis and patients with mild-to-moderate stenosis (p>0.05 for both). Correlation analysis demonstrated a negative correlation between LMR and mean aortic pressure gradient (r=-0.232, p=0.002). Linear regression analysis, performed in order to identify independent variables related to the mean gradient in severe aortic stenosis, revealed increased NLR and decreased LMR as factors that were independently related to the severity of aortic stenosis (p=0.028 and p=0.003, respectively; Table 3).
Echocardiographic characteristics of the study participants.
| Severe CAS (n=67) | Mild-to-moderate CAS (n=111) | Controls (n=139) | p* | p† | pδ | p§ | |
|---|---|---|---|---|---|---|---|
| Maximum gradient (mmHg) | 78.55±19.71 | 32.99±10.9 | <0.001 | ||||
| Mean gradient (mmHg) | 48.63±10.29 | 22.11±7.11 | <0.001 | ||||
| Ejection fraction (%) | 61.18±6.00 | 59.80±5.91 | 61.06±5.85 | 0.289 | 0.991 | 0.215 | 0.124 |
| Left atrium (mm) | 38.93±4.39 | 35.70±4.44 | 3.40±4.68 | <0.001 | <0.001 | 0.015 | <0.001 |
| Septal wall (mm) | 12.9±1.09 | 11.3±1.35 | 10.3±1.19 | <0.001 | <0.001 | <0.001 | <0.001 |
| LVEDD (mm) | 51.37±5.14 | 49.59±4.51 | 47.50±3.38 | 0.018 | <0.001 | <0.001 | <0.001 |
| LVESD (mm) | 34.61±4.34 | 32.62±4.00 | 31.7±3.44 | 0.003 | <0.001 | 0.144 | <0.001 |
CAS: calcific aortic stenosis; LVEDD: left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter.
Multivariate linear regression analysis showing independent predictors of calcific aortic stenosis severity (mean aortic gradient).
| Variables | Univariate regression analysis | Multivariate regression analysis | ||
|---|---|---|---|---|
| Beta (95% CI) | p | Beta (95% CI) | p | |
| Hypertension | -0.232 (-4326 to -1.007) | 0.002 | ||
| WBC | 0.134 (-0.099 to 1.992) | 0.076 | ||
| Neutrophils | 0.117 (-0.119 to 0.409) | 0.277 | ||
| Lymphocytes | -0.236 (-9.062 to 0.375) | 0.022 | ||
| Monocytes | 0.214 (0.637 to 3.316) | 0.004 | ||
| LMR | -0.188 (-1.305 to 10.389) | 0.012 | -0.218 (-2.287 to 11.245) | 0.003 |
| NLR | 0.201 (1.969 to 12.387) | 0.007 | 0.349 (1.352 to 23.572) | 0.028 |
| Creatinine | 0.139 (-0.524 to 18.239) | 0.064 | ||
CI: confidence interval; LMR: lymphocyte/monocyte ratio; NLR: neutrophil/lymphocyte ratio; WBC: white blood cell count.
Our findings suggest that LMR is associated with and can be used as a predictor for the severity of CAS. To the best of our knowledge, this study is the first of its kind to assess the relationship between LMR and severity of CAS.
CAS and atherosclerosis are similar in many respects. As in atherosclerosis, the pathophysiology of CAS includes chronic inflammation, lipoprotein accumulation, fibrosis, and calcification.11,12 Valvular endothelial injury resulting from increased mechanical stress and decreased shear stress triggers lipid penetration and accumulation, while lipid accumulation and oxidation induce inflammation.1 Inflammatory cells including monocytes, macrophages, and lymphocytes accumulate in the injured tissue, secrete various pro-inflammatory cytokines and induce a series of pathological processes resulting in valvular fibrosis and calcification.1,11,13 Some studies indicate that lymphocyte count was associated with a poor cardiovascular outcome and that a decreased lymphocyte level was an independent risk factor for coronary artery disease.14,15 Moreover, others have revealed that patients with CAS have lower numbers of lymphocytes compared to those without.16 Similarly, in the development of atherosclerosis, monocytes that reach the target tissue transform into macrophages and remove noxious molecules such as low-density lipoprotein (LDL).17 Monocytes were shown to be independent and important indicators of plaque formation and progression in atherosclerosis18; they have also been shown to play an important role in the development of atherosclerotic plaques by secreting pro-inflammatory cytokines such as platelet-derived growth factor, interleukin 1, and interleukin 6.17 Additionally, elevated monocyte levels were shown to be associated with increased risk for coronary artery disease.19
As mentioned above, inflammation plays a pivotal role in the development of both coronary artery disease and CAS. Previous studies have shown that markers of systemic inflammation are also associated with atherosclerosis and coronary artery calcification.20,21 Similarly, several reports in the literature suggest that systemic inflammation is closely associated with CAS and that certain inflammatory markers have the potential to serve as predictors for the severity, progression, and prognosis of CAS.22 It has been shown that the NLR, which was recently demonstrated to be a marker of systemic inflammation, could also be correlated with the severity of the aortic stenosis process in patients diagnosed with CAS.23,24
Recently, a large body of evidence has suggested that the LMR, as well as being a marker of inflammation, can function as a useful prognostic marker in cancer patients.23,25 Other recent studies have also demonstrated that the LMR, as an indicator of increased inflammatory status, is associated with several cardiovascular diseases. Murat et al. observed that the LMR was inversely related to the development of in-stent restenosis.9 A study on patients with peripheral arterial disease reported that decreased LMR was closely associated with critical limb ischemia and other vascular endpoints.26 Another study, on patients hospitalized for cardiac failure and then discharged, showed that decreased LMR was related to increased risk for six-month mortality.10 Although it is difficult to say whether reduced LMR is a cause or a consequence of CAS, data from the present study clearly reveal that the LMR is significantly decreased with greater severity of aortic stenosis and is an independent indicator for the severity of the aortic stenosis process. Considering the relationship between inflammation and aortic stenosis, the results of our study are similar to those in previous reports.
Aortic calcification has been shown to share the same risk factors as atherosclerosis, which suggests a potential benefit from statin therapy. But it has been demonstrated that the LDL cholesterol-lowering effect of statins does not significantly reduce AS progression.27 There were no significant differences in terms of LDL values between groups in our study.
Although inflammation plays a pivotal role in the generation and progression of CAS, many other factors such as lipid accumulation, lipoprotein oxidation, matrix metalloproteinase activity, and local production of proteins like osteopontin (involved in tissue calcification) may also contribute to this process.1,28–30 In addition, genetic predisposition has recently been put forward as a risk factor for CAS.31 Considering the multifactorial and complex pathophysiology of CAS, inflammation and inflammatory markers may play a relatively limited role in this process. This may explain why the correlation that we found between the level of CAS severity and the LMR is relatively weak.
LimitationsThe study suffered from certain limitations that need to be highlighted, including the fact that it was an observational, retrospective and single-center study. In addition, the absence of comparison between the LMR and other inflammatory markers such as C-reactive protein, and inadequate follow-up of clinical events, should also be mentioned.
ConclusionsIn conclusion, in accordance with the aforementioned studies, inflammatory status, as indicated by the LMR, may be associated with CAS. A simple parameter that is easy to compute, LMR, by virtue of its relation with inflammation, has tremendous potential as a marker for predicting the severity of CAS.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.