Syncope is defined as a transient loss of consciousness due to global cerebral hypoperfusion and is one of the leading causes of emergency department admission. The initial approach should focus on excluding non-syncopal causes for loss of consciousness and risk stratification for a cardiac cause, in order to ensure an appropriate etiological investigation and therapeutic approach. Vasovagal syncope (VVS), the most common type of syncope, should be assumed once other causes are excluded. Pathophysiologically, the vasovagal reflex is the result of a paradoxical autonomic response, leading to hypotension and/or bradycardia. VVS has not been shown to affect mortality, but morbidity may be considerable in those with recurrent syncopal episodes. The management of VVS includes both non-pharmacological and pharmacological measures that act on various levels of the reflex arc that triggers the syncopal episode. However, most are of uncertain benefit given the scarcity of high-quality supporting evidence. Pacemaker therapy may be considered in recurrent refractory cardioinhibitory reflex syncope, for which it is currently considered a robust intervention, as noted in the European guidelines. Non-randomized and unblinded studies have shown a potential benefit of pacing in recurrent VVS, but double-blinded randomized controlled trials have not consistently demonstrated positive results. We performed a comprehensive review of the current literature and recent advances in cardiac pacing and pacing algorithms in VVS, and discuss the diagnostic and therapeutic approach to the complex patient with recurrent VVS and reduced quality of life
A síncope define-se como uma perda transitória do conhecimento devido a hipoperfusão cerebral global e representa uma das principais causas de vinda ao Serviço de Urgência. Na abordagem inicial do doente neste contexto é fundamental estratificar o risco para síncope de causa cardíaca, promovendo um adequado estudo etiológico e orientação terapêutica. A etiologia mais comum é o reflexo vasovagal, o qual parece resultar de uma resposta autonómica paradoxal com consequente hipotensão e/ou bradicardia. Ainda que a síncope vasovagal seja uma condição sem impacto na mortalidade, esta frequentemente afeta uma população jovem e causa uma morbilidade significativa, sobretudo quando associada a uma elevada taxa de recorrência. Na abordagem terapêutica da síncope incluem-se estratégias não-farmacológicas comportamentais e terapêuticas farmacológicas que atuam nos vários níveis do arco reflexo desencadeante do episódio sincopal. Contudo, ambas são suportadas apenas por evidência de robustez limitada. Nos casos em que estas intervenções se mostram insuficientes, o uso de pacemaker definitivo tem sido proposto como estratégia terapêutica, agora com maior força de robustez nas atuais Recomendações europeias. Os estudos iniciais não-aleatorizados e sem ocultação demonstravam um potencial benefício de tal intervenção, com redução da recorrência de episódios sincopais. Contudo, os estudos aleatorizados e de dupla ocultação têm resultados díspares. Tendo por base as diferenças destes estudos, os autores efetuaram uma revisão abrangente da literatura acerca da evidência do pacing cardíaco e respetivos algoritmos e quais os fatores a considerar na decisão diagnóstica e terapêutica individualizada, no doente com síncope vasovagal recorrente.
Syncope is defined as a transient loss of consciousness due to global cerebral hypoperfusion characterized by short duration and spontaneous recovery.1,2 It is one of the leading causes of emergency department admission, accounting for 1-3% of all cases, followed by hospitalization for etiological investigation in around 40% of these cases.3,4 The vasovagal or neurocardiogenic reflex is the most common cause of syncope5 and vasovagal syncope (VVS) can lead to considerable morbidity, even though it has not been shown to affect mortality.6
Reflex syncope is an important issue due to its high prevalence, incidence and associated morbidity, together with significant gaps in knowledge concerning the appropriate therapeutic approach. This review discusses the following issues: the pathophysiological mechanisms behind reflex syncope, particularly cardioinhibitory and vasodepressor responses; the definition of loss of consciousness and differential diagnosis of syncope; differential diagnosis of atrioventricular (AV) block of extrinsic and intrinsic cause; and a brief review of therapeutic approaches, particularly the potential role of pacemaker therapy.
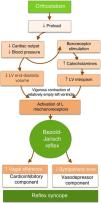
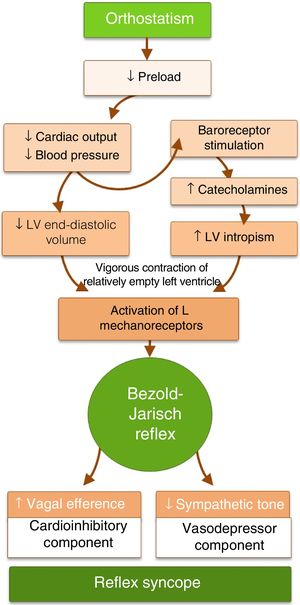
PathophysiologyThe mechanism underlying reflex syncope is a paradoxical autonomic reflex that leads to hypotension (vasodepression) and/or bradycardia (cardioinhibition) (Figure 1). It begins with a specific stimulus that triggers the afferent pathway and culminates in an efferent response that leads to an increase in parasympathetic tone and/or in inhibition of sympathetic tone. In some circumstances, such as as carotid sinus hypersensitivity, the initial stimulus can be identified, while in others it is unknown.
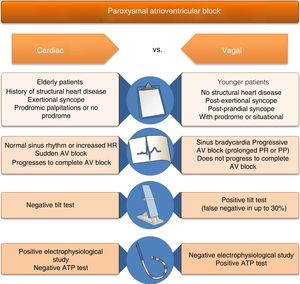
Reflex syncope is often associated with orthostatic stress.7 Orthostatism reduces venous return and hence cardiac output, blood pressure (BP), and end-diastolic volume. The reflex arc that maintains normal cerebral perfusion and prevents hypotension or syncope with orthostatic changes consists of three components. In response to lowered BP, baroreceptors in the carotid sinus, aortic arch and left ventricle send afferent stimuli to the vasomotor center in the medulla oblongata. Integration of this response results in efferent stimuli that increase sympathetic tone and circulating catecholamines and reduce parasympathetic tone, leading to vasoconstriction and positive chronotropic and inotropic effects. Triggers of the paradoxical response in patients with VVS appear to be reduced left ventricular end-diastolic volume and increased catecholamine secretion. Vigorous contraction of a relatively empty left ventricle, triggered by catecholaminergic action, stimulates mechanoreceptors in the ventricle (unmyelinated C-fibers found in all four cardiac chambers). This sends afferent impulses to the dorsal nucleus of the vagus nerve in the medulla, giving rise to an efferent impulse that paradoxically reduces sympathetic tone and/or increases parasympathetic tone, with vasodepressor (hypotension) and/or cardioinhibitory (bradycardia) effects, respectively (the Bezold–Jarisch reflex).8 Hypotension and bradycardia, resulting in cerebral hypoperfusion, lead to loss of consciousness. Since both the sinoatrial and AV nodes are heavily innervated with autonomic nerves, bradycardia in the Bezold-Jarisch reflex may be due to increased vagal stimulation, decreasing the automaticity of the sinoatrial node and reducing conduction in the AV node, leading to AV block (Figure 2 and Table 1). As both vagal hyperactivity (extrinsic) and conduction system disease (intrinsic) can cause AV block and syncope, these must be distinguished due to their different therapeutic and prognostic implications.
Differential diagnosis of atrioventricular block due to extrinsic and intrinsic causes, based on clinical history, physical examination and diagnostic exams.
| AV block of intrinsic cause (alarm signs suggesting cardiac etiology) | AV block of extrinsic cause (no alarm signs, suggesting reflex etiology) |
|---|---|
| Clinical history | |
| First syncopal episode at older ageKnown structural heart diseaseFamily history of sudden cardiac deathSyncope without prodrome (or short prodrome with palpitations)Exertional syncopeSyncope while sitting or lying |
|
| ECG | |
|
|
|
|
|
|
AF: atrial fibrillation; AV atrioventricular: ECG: electrocardiogram; LAFB: left anterior fascicular block; LBBB: complete left bundle branch block; RBBB: complete right bundle branch block.
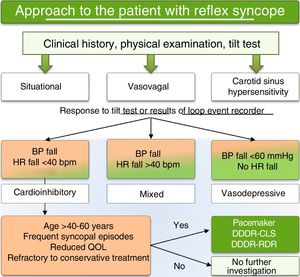
Reflex syncope can result from a purely vasodepressor effect or, less frequently, a solely cardioinhibitory response,9 depending on whether the efferent response inhibits sympathetic tone or increases parasympathetic tone, respectively. In the VASIS trial,10 the cardioinhibitory response was divided into two types, 2A (defined as heart rate [HR] rising initially then falling to a ventricular rate <40bpm for >10s or asystole occurring for >3s, with BP rising initially then falling before HR falls), and 2B (defined as the same behavior but with BP only falling to hypotensive levels <80mm Hg systolic BP at or after the onset of rapid and severe HR fall). A mixed response, in which hypotension usually precedes bradycardia, appears to be the most common form.
Identifying the signals that trigger these efferent responses has proved to be a challenge, and research in humans has been limited.7,11 Largely on the basis of animal studies, there appears to be more than one underlying mechanism. Small case-control studies on patients with a positive tilt test and simultaneous echocardiographic monitoring have shown reduced left ventricular volume during the test, supporting the concept of the ‘empty ventricle’.12,13 On the other hand, similar studies have found no evidence of hypercontractility or increased left ventricular systolic pressure at the beginning of tilt testing,14 which suggests that mechanical stimulation of the left ventricle is not necessarily a component of the reflex arc in all forms of VVS.14 Interruption of ventricular C-fiber afferents in animals was shown to prevent hypotension and bradycardia in response to acute hemorrhage. However, a similar experimental study in dogs did not reproduce this result.11 Furthermore, there are reports of heart transplantation patients who subsequently suffered VVS even though there was no evidence of significant reinnervation of the transplanted heart, which means that in these patients autonomic stimulation could not have been a cause of syncope.15,16 Although most of these studies have been in animals and there is very little evidence from human studies, ventricular afferents do not appear to be absolutely necessary for the development of VVS; there may be other cardiovascular structures that cause VVS if stimulated as a result of hypovolemia, although there is as yet no experimental evidence for this theory.17
Although the Bezold–Jarisch reflex can explain hypotension and bradycardia in some cases, there must be other mechanisms that are responsible for recurrent VVS in other patients. Studies of alterations in neurotransmitters such as endogenous opioids, nitric oxide,11 adenosine18 or serotonin19 suggest that they may inhibit sympathetic responses. Central or peripheral baroreceptor reflex abnormalities have been proposed as another possible pathophysiological mechanism.17
With regard to hemodynamic responses, in their review article, Jardine et al.20 proposed that there is a sequence of hemodynamic changes, the ‘four phases of syncope’, that are common to all patients with VVS. In phase 1, termed early stabilization, a fall in venous return results in reduced systolic volume and cardiac output despite an increase in HR; mean arterial pressure is maintained by an increase in systemic vascular resistance. Phase 2, circulatory instability, occurs when there is an additional increase in lower body negative pressure (LBNP), exacerbating the effects seen in phase 1. In phase 3, terminal hypotension, as LBNP is increased, HR and cardiac output fall, BP variability virtually disappears and a classic VVS episode occurs. Phase 4 (recovery) occurs rapidly after cessation of LBNP.20
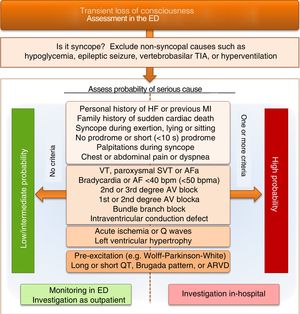
Differential diagnosis of vasovagal syncopeFor patients with loss of consciousness in an urgent setting, VVS should be a diagnosis of exclusion after appropriate etiological investigation has ruled out other causes. Figure 3 presents a proposed simplified algorithm for the differential diagnosis of a patient with loss of consciousness admitted to the emergency department. The algorithm stresses the importance of prompt exclusion of common conditions that must be differentiated from the syncopal episode itself.21 It also focuses on risk stratification for cardiogenic syncope, which is a simple way to identify the best approach to investigation and treatment of these patients. Although a definitive diagnosis is difficult to obtain in an urgent setting, such stratification is essential to enable low-risk patients to be appropriately and safely investigated as outpatients and for high-risk patients to receive prompt monitoring, investigation and treatment in an in-hospital setting.1,2
Differential diagnosis of loss of consciousness and syncope on admission to the emergency department, focusing on risk stratification for cardiogenic syncope. AF: atrial fibrillation; ARVD: arrhythmogenic right ventricular dysplasia; AV: atrioventricular; ED: emergency department; HF: heart failure; MI: myocardial infarction; SVT: supraventricular tachycardia; TIA: transient ischemic attack; VT: ventricular tachycardia. aonly to be considered if history suggests arrhythmic syncope.
In most high-risk patients with criteria for hospitalization, syncope is suspected to be of cardiogenic cause, either structural or arrhythmic. In the latter case, the implantable loop event recorder has become an indispensable diagnostic tool, especially when the cause of syncope is unknown. Published studies22–25 have shown that implantable event recorders are more likely to diagnose the cause of syncope than Holter ECG monitoring, external event recorders, tilt testing or electrophysiological study. Although implantable event recorders are the preferred monitoring method in an outpatient setting, the European Society of Cardiology (ESC) guidelines22 state that Holter monitoring or an external event recorder may be used in patients whose symptoms occur less than weekly or monthly, respectively.
Therapeutic approach to the patient with reflex syncopeInitial non-pharmacological therapyThe first step in the approach to patients with VVS is to educate them, beginning by explaining the benign nature of the condition and instructing them on how to avoid triggering factors such as prolonged standing, dehydration and extremes of temperature.1,2 Isometric physical counterpressure maneuvers such as handgrip, leg crossing or squatting significantly increase BP and, especially in patients with long prodromes who can recognize the symptoms of presyncope early, can delay or even prevent loss of consciousness.26–28 A multicenter clinical trial showed that such measures, when performed immediately after the onset of the prodrome, reduced the relative risk (RR) of recurrence by 39%. However, 35% of randomized subjects did not use the maneuvers, because of either absence of any warning or too short duration of prodromal symptoms.29 Furthermore, such maneuvers may not be effective in older patients with reduced muscular strength. Although the above randomized trial is the only one to study the benefit of physical counterpressure maneuvers, their harmlessness and low cost make them a valid option to be considered in the initial approach to VVS,30 with class of recommendation IIa for younger patients in the guidelines.22
Measures that increase venous return, such as volume expansion by drinking up to 3l of fluids a day and ingesting 10g/day of salt, and wearing elastic graded compression stockings, can be used if not contraindicated, although evidence for their efficacy is limited.1,2,28
Another option is orthostatic training, of which there are two main forms: tilt training with repeated monitored sessions on a tilt table, and daily standing against a wall at home for prolonged periods.30 In a non-randomized trial including 47 adolescents, tilt training followed by standing at home reduced recurrence of syncope at 18-month follow-up.31 However, since this method requires a high degree of motivation, long-term adherence and thus efficacy are often poor.32 Doubts concerning its effectiveness also persist due to its failure to reduce syncope recurrence in other studies.33–35 Overall, the currently available evidence is insufficiently robust to recommend orthostatic training as a matter of routine.
Cardioneuroablation consists of the radiofrequency ablation of ganglionated plexuses on the endocardial surface. Non-randomized studies with small study populations have suggested benefits in reducing recurrence of syncopal episodes,36,37 which was confirmed in a recent literature review.38 However, there is currently little evidence on the efficacy of this technique.36,37
Psychotherapeutic measures may be a helpful component of treatment in patients with recurrent VVS, who frequently suffer from anxiety and negative psychosocial effects. Such measures may have a synergistic effect on the response to other therapies.39
Finally, a thorough review of the patient’s usual medication is essential. Reducing the dosage or discontinuing drugs that can potentially induce syncope, such as vasodilators and diuretics, is an important measure for preventing or reducing the likelihood of future syncopal events.22
Pharmacological therapyPharmacological therapy may be considered in patients with recurrent syncope that has a significant impact on their quality of life and is associated with a high risk of trauma, as well as those whose activities (such as driving, flying, or competitive athletics21) involve high risks to themselves or to others, when the condition has proved refractory to the non-pharmacological measures described above. Various drugs, acting at different points of the reflex arc, have been investigated for the treatment of VVS.28
Beta-blockersBeta-blockers act on the afferent limb of the reflex arc involved in VVS. Their efficacy has been tested in various randomized clinical trials, mostly on metoprolol, pindolol and atenolol.40–44 The largest randomized controlled double-blinded trial (POST43) showed that metoprolol was not effective in preventing syncope recurrence at one-year follow-up. However, a subsequent meta-analysis that included the POST trial concluded that beta-blocker therapy can be beneficial in patients aged ≥42 years of age but not in those aged <42 years.30 Several small studies have assessed different beta-blockers, analyzing different outcomes such as tilt test results or syncope recurrence, with conflicting results.40,41,45–47 In the absence of robust evidence in favor of beta-blockers, and given the negative results of two randomized double-blinded trials34,36 together with the known adverse side-effects of these drugs, they are contraindicated for prevention of VVS in patients of all ages in the current guidelines (class III recommendation).22 The randomized, triple-blinded POST 5 trail (ClinicalTrials.gov identifier NCT02123056) aims to determine if treatment with metoprolol in patients ≥40 years old will better suppress syncope recurrences than placebo. Its results are awaited.
MidodrineVasopressor agents counteract the vasodepressive effect that in VVS arises as a result of reduced sympathetic tone. Midodrine, an alpha-adrenergic agonist, is frequently used in this context. In a meta-analysis including 115 patients in four studies, syncope recurrence was lower and quality of life was better during midodrine treatment than with non-pharmacological therapy or placebo.48 A subsequent meta-analysis that included 593 patients suggested that midodrine improves clinical outcomes in patients with recurrent VVS, although the evidence was of low or moderate quality.49
Results are awaited from the POST 4 trial (ClinicalTrials.gov identifier NCT01456481), a randomized clinical trial designed to assess the efficacy of midodrine compared to placebo in preventing syncope recurrence. Currently available evidence indicates that midodrine may be considered for patients with VVS requiring pharmacological therapy.1,2 Its main disadvantages are its short half-life, requiring three doses a day, and side-effects that include urinary retention and supine hypertension, which particularly limit its utility in older patients.39
FludrocortisoneFludrocortisone, a mineralocorticoid, causes fluid retention and a slight increase in BP. The multicenter randomized controlled POST 2 trial in 210 patients compared the efficacy of fludrocortisone in reducing recurrent VVS with placebo over a one-year treatment period.50 The benefit demonstrated was only a statistical tendency in favor of fludrocortisone, but when the analysis was restricted to outcomes after two weeks of dose stabilization, there was a significant reduction in syncopal episodes in the treatment arm. It should be noted that the median age of the patients enrolled was 30 years and that those with hypertension were excluded. Fludrocortisone should thus be reserved for patients with repeated episodes of VVS and without contraindications to its use, particularly hypertension. Besides supine hypertension and weight gain, the main adverse effects are nausea and hypokalemia.30
Serotonin uptake inhibitorsSerotonin uptake inhibitors such as paroxetine, sertraline and fluoxetine may be of benefit for the treatment of VVS in patients who do not respond to or do not tolerate other drugs.19,45,51 In a recent randomized placebo-controlled trial of 106 patients with recurrent VVS, scoring positive on the Anxiety Sensitivity Index and not diagnosed with psychiatric disease, the number of patients with recurrent syncope or presyncope during a one-year follow-up was lower in the fluoxetine group.45 However, the benefit of this drug class is still uncertain and its use is not recommended due to the scarcity of available evidence.30
IvabradineIn a non-randomized, non-controlled trial published in 2014 including 25 patients with postural orthostatic tachycardia syndrome, ivabradine demonstrated some benefit in reducing episodes of VVS.52 However, evidence of its benefit in these patients is lacking and further research should be undertaken.
Use of pacemakers to treat cardioinhibitory reflex syncopeAccording to the most recent ESC guidelines, implantation of a permanent pacemaker (PPM) should be considered in patients aged >40 years with cardioinhibitory reflex syncope with symptomatic asystolic pause(s) >3s or asymptomatic pause(s) >6s or with cardioinhibitory carotid sinus syndrome with recurrent frequent unpredictable syncope (class IIa recommendation).22 The class of recommendation was upgraded based on a review of previously available evidence and recent studies.
It should be borne in mind that the main studies on VVS have different inclusion and exclusion criteria, age of patients included, definition of the cardioinhibitory response, population characteristics and results. The following sections focus on detailed comparisons between randomized trials (Table 2).
Summary of evidence from trials investigating permanent pacing compared to placebo for treatment of vasovagal syncope that randomized more than 20 patients.
| Trial | VPS I (Connolly et al.53) | VASIS-PM (Sutton et al.10) | VPS II (Connolly et al.58) | SYNPACE (Raviele et al.59) | ISSUE-3 (Brignole et al.55) | Russo et al.56 | SPAIN (Baron-Esquivias et al.57) |
|---|---|---|---|---|---|---|---|
| Center(s) | Multicenter | Multicenter | Multicenter | Multicenter | Multicenter | Multicenter | Multicenter |
| Study design | Randomized | Randomized | Randomized | Randomized | Randomized, 12-month crossover | Randomized, 18-month crossover | Randomized |
| Blinded | No | No | Double | Double | Double | Single | Double |
| No. of patients | 54 | 42 | 100 | 29 | 77 | 50 | 46 |
| PPM arm vs. placebo arm | 27 vs. 27 | 19 vs. 23 | 48 vs. 52 | 16 vs. 13 | 38 vs. 39 | NI | 21 vs. 25 |
| Placebo arm | No intervention | No intervention | PPM OFF | PPM OFF | PPM OFF | PPM OFF | Sham PPM |
| Mean follow-up | NI | 3.76±2.2 years | 6 months | 715 (302–785) days | 24 months | 36 months | 22.2±5.1 months |
| Inclusion criteria | |||||||
| Type of syncope | Vasovagal | Vasovagal | Vasovagal | Vasovagal | Vasovagal | Vasovagal | Vasovagal |
| No. of syncopal episodes | ≥6 | ≥3 in the last 2 years | ≥3 in the last 2 years | ≥6 | ≥3 in the last 2 years | ≥2 | ≥5 |
| Last syncopal episode | – | <6 months | – | <6 months | – | – | ≥2 in the last year |
| Time between first and last episode | – | >6 months | – | – | ≥1 month | – | – |
| Criteria of cardioinhibition with reproduction of syncope | HR<60 bpm if no isoproterenol used, or <70 bpm if ≤2μg/min or <80 bpm if >2μg/min of isoproterenol | HR<40 bpm for >10s or asystole >3s with BP fall | HR×BP product <6000 | HR<60 bpm for >10s or asystole ≥3s | Asystole >3s with syncope or >6s with presyncope or asymptomatic | Asystole >3s with BP fall | HR<40 bpm for >10s or asystole >3s with BP fall |
| Detection of cardioinhibition | TT | TT | TT | TT | ILR | TT | TT |
| Intervention | PPM vs. placebo | PPM vs. placebo | PPM ON vs. PPM OFF | PPM ON vs. PPM OFF | PPM ON vs. PPM OFF | PPM ON vs. PPM OFF | PPM vs. sham DDI |
| PPM programming | DDI-RDR | DDI-RDR | DDD-RDR | DDD-RDR | DDD-RDR | DDD-CLS | DDD-CLS |
| Other | – | Age >40 years or <40 years if refractory to drug therapy | Age >19 years | Age >18 years and ≥1 syncope within 12 months of positive TT | Age >40 years, refractory to therapy | Age >40 years, SR | Age >40 years with normal ECG |
| Exclusion criteria | |||||||
| Pure vasopressor syncope | Excluded | Excluded | Includedb | Excluded | Excluded | Excluded | Excluded |
| Other key criteria | Severe chronic disease, CSH, significant valve disease | MI <6 months, HF (NYHA III-IV), severe chronic disease | Abnormal ECG, severe chronic disease | MI <6 months, HF (NYHA III-IV), severe chronic disease, CSH | HSC, new-onset HF, LVEF≤40%, MI | Hypertension, diabetes, HF, anemia, CAD | CSH |
| Population characteristics (PPM arm) | |||||||
| Mean age, years | 46±18ª | 64±11a | 50±18 | 52±19 | 63±14 | 53±5 | 57±13 |
| Male | 30% | 58% | 27%a | 31% | 53% | 66% | 48% |
| Mean previous syncopal episodes | 14 (8–35) | 5 (3–12) | 15 (8–50) | 14 (9–30) | 7 (4–12) | 7±3 | 12 (9–20) |
| Presyncope | NI | 63% | 71% | 3 (0–10) | 50% | NI | NI |
| Asystole >3s | 0% | 86% | 0% | 52% | 100% | 100% | 76% |
| History of trauma | NI | 42% | 13% | 25% (major) | 5% (major)39% (minor) | 8% | NI |
| Outcomes | |||||||
| Recurrence of syncope (PPM vs. placebo) | 22% vs. 70%a | 5% vs. 61%a | 31% vs. 40% | 50% vs. 38% | 25% vs. 57%a | 2 vs. 15a | 8.7% vs. 45.7%a |
BP: blood pressure; CAD: coronary artery disease; CLS: closed-loop stimulation; CSH: carotid sinus hypersensitivity; ECG: electrocardiogram; HF: heart failure; HR: heart rate; ILR: implantable loop recorder; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NI: no information; NYHA: New York Heart Association functional class; PPM: permanent pacemaker; RDR: rate-drop response; SR: sinus rhythm; TT: tilt test.
The potential of permanent pacing to reduce syncope recurrence was initially assessed in trials in which the evidence was weakened by flaws in study design. Of these, the non-blinded randomized VPS I, VASIS and SYDIT trials will be discussed below.
VPS I (n=54) was the first randomized trial to investigate the role of permanent pacing in VVS with syncope or presyncope reproducible on tilt testing and demonstration of relative bradycardia. In this trial, there was a significant reduction in risk of syncope in patients who received a PPM with a rate-drop response (RDR) algorithm compared to placebo (22% vs. 70%; relative risk reduction [RRR] 0.85; 95% confidence interval [CI] 0.60-0.95; 2p=0.000022) at one year.53
The VASIS trial of 42 patients with cardioinhibitory VVS showed a statistically significant reduction in syncope recurrence in the DDI pacemaker arm compared to placebo (5% vs. 61%; RR 0.04; 95% CI 0.005-0.3; p=0.0006) in a mean follow-up of 3.76±2.2 years. Syncope recurrence was recorded in 61% of patients who did not receive a PPM, with a mean of 1.76±0.9 episodes per patient (0.44 episodes per year). Not only was recurrence rare in the PPM arm, but no syncopal episodes were associated with traumatic injury.10
The multicenter prospective SYDIT trial included patients aged >35 years with recurrent VVS (≥3 episodes in the previous two years) and a positive tilt test for syncope in association with relative bradycardia (HR<60 bpm). Participants were randomized to receive a DDD PPM with RDR function (n=46) or atenolol 100mg/day (n=47) and followed for a mean of 520±266 days. In 56.0% of patients tilt-induced syncope was observed only after pharmacological provocation and a pure cardioinhibitory response to tilt testing (with asystole >3s) was present in only 60.2%. Although this study did not have a placebo arm, and notwithstanding the limitations inherent to the study design, syncope recurrence was reduced with permanent pacing compared to medical treatment (4.3% vs. 25.5%, odds ratio [OR] 0.133; 95% CI: 0.028-0.632; p=0.004). The authors concluded that, in this population with a mean age >50 years and a high prevalence of cardioinhibitory syncopal episodes, DDR pacing was superior to pharmacological beta-blockade in reducing syncope recurrence.54
Blinded randomized trialsThe non-blinded randomized trials described above indicated that permanent pacing could benefit selected patients. However, their study design has significant limitations and is subject to bias. Researchers accordingly proceeded with blinded randomized trials, with mixed results: some favored the use of pacing (ISSUE-3, Russo et al., SPAIN), while others showed no benefit (SYNPACE, VPS II).
The ISSUE-3 trial, in 77 patients aged ≥40 years who had experienced prolonged asystole (>3−6s) and who received a DDD pacemaker, showed a statistically significant reduction in recurrence of cardioinhibitory VVS in the PPM ON group compared to the PPM OFF group (25% vs. 57%; RR 0.57; 95% CI 0.40-0.74; p=0.04) in a two-year follow-up. Although the trial demonstrated the efficacy of permanent pacing in this population, it should be noted that syncopal recurrence was 25% in the PPM ON group, which is higher than in the non-blinded studies described above. The authors attribute this to the likelihood of a vasodepressive component contributing to the mechanism of some syncopal episodes.55
The study by Russo et al. included 50 patients with cardioinhibitory VVS (asystole >3s) who received a DDR pacemaker with closed-loop stimulation (CLS) and were randomized to CLS OFF or ON. Patients in the ON group had fewer syncopal episodes (2 vs. 15; p=0.007) over a 36-month follow-up.56
In the SPAIN trial (n=46), a crossover study with either DDD pacing with CLS or sham DDI pacing, there was a significantly greater number of patients with ≥50% reduction in the number of syncopal episodes in the former group (72% vs. 28%; p=0.017). Over the course of the study, syncopal events were less frequent in the DDD-CLS group than in the sham group (8.7% vs. 45.7%; hazard ratio 6.7; 95% CI 2.3–19.8) in a mean follow-up of 22.2±5.1 months. Following crossover in the second year, syncope recurred in 29% of those who had previously received DDD-CLS, while all the patients who crossed over from sham to treatment had a ≥50% reduction in the number of syncopal episodes (p=0.0003). These findings increased the weight of evidence in favor of this intervention.57
By contrast, in the VPS II trial (n=100), no reduction in cumulative risk of syncope was seen in patients who received DDD pacing with RDR compared to the PPM OFF group (31% vs. 40%; RRR 0.30; 95% CI -0.33-0.63; p=0.14) in a six-month follow-up.58 Similarly, the SYNPACE study (n=29) failed to show statistically significant differences in outcomes between the group receiving DDD pacing with RDR and the PPM OFF group, including in subgroup analysis (cardioinhibitory and mixed response), after a median 715-day follow-up. A cardioinhibitory response was defined as syncope reproducible on tilt testing associated with asystole ≥3s or HR<60 bpm. Tilt testing was only positive after administration of nitroglycerin in 69% of patients. An asystolic response was present in 52% of patients and a mixed response in 48%.59
The results of these studies have been the subject of considerable debate. Possible reasons put forward for the apparent disparities include differences between studies in inclusion criteria, study design and PPM programming in the intervention arm, as well as a genuine absence of benefit of permanent pacing in VVS. Importantly, the positive studies tended to include older patients, which raises questions concerning the effect of age on the different pathophysiological mechanisms underlying VVS. Furthermore, in the SYNPACE trial, the criteria for mixed-response syncope were relatively broad, covering patients with HR<60 bpm (as opposed to the usual <40 bpm) for >10s, and these patients represented 48% of the study population.59 These criteria may have led to the inclusion of patients with syncope of predominantly vasodepressive pathophysiology. Similarly, the VPS II trial included patients with predominantly vasodepressive syncope, since the mean lowest HR recorded was 53 bpm and only 19% of patients had HR<40 bpm, in contrast to the inclusion criteria of the positive studies, which were more restrictive concerning a cardioinhibitory response.
The diagnostic value of tilt testing for accurate diagnosis of a cardioinhibitory response as a cause of syncope has been questioned.60 However, current evidence indicates that a cardioinhibitory response during tilt testing predicts asystolic syncope with high probability, and on this basis, the latest ESC guidelines recommend tilt testing as essential in the diagnostic algorithm for asystole, guiding the decision to use pacemaker therapy, particularly in patients aged >40 years with recurrent cardioinhibitory syncope.22
According to a meta-analysis assessing whether pacing reduces risk of reflex syncope, the evidence for its use in this condition is limited.61 Although its analysis of unblinded trials points to lower incidence of syncope with pacing, the only two double-blinded randomized trials included (SYNPACE and ISSUE-3) failed to show a statistically significant effect. The authors suggest that this discrepancy may be the result of bias in the unblinded trials.61 Even so, it would be interesting to update this systematic review to include the recently published SPAIN trial, which satisfied all the requirements for inclusion criteria discussed by the authors, and could alter the conclusions of their analysis.
In summary, bearing in mind that reflex syncope does not affect mortality and is frequently found in younger individuals, as well as the risks involved in placement of a PPM and the conflicting evidence on its efficacy, pacing should be reserved for patients who are most likely to benefit from the intervention. The decision process should include assessment of recurrence, the underlying pathophysiological mechanism, and the impact of the condition on the patient’s quality of life, as well as response to therapeutic measures such as physical counterpressure maneuvers. Pacemaker therapy should thus be considered for carefully selected patients aged >40 years with frequent recurrence and documented cardioinhibitory response.
DDD pacing with CLS detects alterations in the contraction dynamics of the right ventricle by measuring changes in intracardiac impedance. If such an alteration is detected in the initial stage, before a syncopal episode begins, the PPM is able to respond early and prevent the syncope from occurring. The benefits of this capability were first seen in small observational studies.62,63 In the INVASY trial (n=50), a single-blind prospective study including patients with recurrent syncope and positive tilt test with cardioinhibition and refractory to conventional measures randomized to DDD pacing with CLS or DDI, no syncope occurred in patients programmed to CLS.64 The study by Russo et al.56 and the SPAIN trial57 described above provided further evidence in favor of this programming algorithm.
Treatment decision algorithmCurrent evidence does not support the routine use of pacing in patients with VVS, but does indicate a role for pacing with RDR or CLS capability in subgroups with frequent recurrence of cardioinhibitory syncope, especially those aged >40-50 years and refractory to other treatment. We accordingly present a treatment decision algorithm for the use of permanent pacing based on current evidence, designed to reduce overuse in patients unlikely to benefit and to avoid underuse in patients for whom the intervention is potentially beneficial (Figure 4). The proposed algorithm is in line with the latest ESC guidelines for the diagnosis and management of syncope,22 which put forward the novel idea that treatment of reflex syncope is better guided by the form of presentation (bradycardia and/or hypotension) than by its etiology. This is in line with the evidence that pacing is only beneficial in certain clinical entities, particularly cardiogenic syncope due to significant bradycardia, cardioinhibitory carotid sinus syndrome, and cardioinhibitory VVS. Based on this reasoning and the results of the ISSUE-3 and SPAIN trials, pacing is a class IIa recommendation in the current ESC guidelines for patients aged >40 years with recurrent cardioinhibitory syncope.22
ConclusionThe therapeutic approach to VVS remains a challenge, particularly in patients whose quality of life is affected by recurrent syncopal episodes and who fail to respond to pharmacological and non-pharmacological measures. Permanent pacing is an option in appropriately selected patients, i.e. those in whom reflex syncope is predominantly cardioinhibitory and who suffer from frequent recurrent syncopal episodes with a significant effect on their quality of life (including a history of major trauma), when other therapeutic options have been exhausted.
FundingThe authors have no funding sources to declare.
Conflicts of interestThe authors have no conflicts of interest to declare.
Authors with equal contribution in carrying out the work.
Please cite this article as: Rocha BML, et al. Abordagem diagnóstica e terapêutica da syncope reflexa cardio-inibitória – A complexidade de um tema controverso. Rev Port Cardiol. 2019;38:661–673.