Technical advances in health care have improved patient survival and quality of life, but are not devoid of complications.
We present the case of a 74-year-old woman with a history of hypertensive heart disease with preserved systolic function, atrial fibrillation and dyslipidemia. She had a DDDR pacemaker implanted in 2005 due to symptomatic complete atrioventricular block.
The patient reported progressive fatigue, weakness, ascites with abdominal discomfort, and lower limb edema, accompanied by non-specific hepatic cholestasis on biochemical testing. Abdominal ultrasound revealed homogeneous hepatomegaly and dilatation of the inferior vena cava and upper hepatic veins, suggestive of congestive hepatopathy.
Echocardiography revealed tricuspid regurgitation progressively worsening over the previous four years and dilatation and progressive dysfunction of the right ventricle, with preserved left ventricular function. The transesophageal echocardiogram revealed severe tricuspid regurgitation with flail septal leaflet and marked dilatation of the tricuspid annulus due to mechanical interference of the pacemaker lead, which was adhering to the septal leaflet. Minimally invasive surgical treatment was performed with partial resection of the leaflet, placement of a tricuspid annuloplasty ring and replacement of the pacemaker lead.
Regression of the congestive symptoms was observed, and the postoperative echocardiogram showed the tricuspid annuloplasty ring with no evidence of stenosis and only slightly dilated right chambers with moderate pulmonary hypertension. Six months after the procedure, the patient suffered an acute neurological event and died.
O progresso técnico associado aos cuidados de saúde tem permitido uma melhoria da sobrevida e da qualidade de vida dos doentes, não sendo, no entanto, isento de complicações.
Apresenta-se o caso de uma doente de 74 anos, com história de cardiopatia hipertensiva com função sistólica preservada, fibrilhação auricular e dislipidemia. Era portadora de pacemaker DDDR desde 2005 por bloqueio auriculo-ventricular completo sintomático.
Referia quadro arrastado de agravamento progressivo de cansaço, astenia, ascite com epigastralgia, e edema dos membros inferiores, acompanhado de colestase hepática bioquímica inespecífica. A ecografia abdominal revelou hepatomegalia homogénea e dilatação das veias cava inferior e supra-hepáticas, sugestivos de hepatite de estase.
Apresentava, ecocardiograficamente, insuficiência tricúspide em agravamento progressivo nos últimos 4 anos, com dilatação e disfunção progressiva do ventrículo direito, mantendo função ventricular esquerda preservada. O ecocardiograma transesofágico revelou regurgitação tricúspide major por flail leaflet do folheto septal, com dilatação marcada do anel valvular, em relação com efeito mecânico do electrocatéter de pacemaker, que se encontrava aderente ao folheto septal. Optou-se por tratamento cirúrgico minimamente invasivo, com ressecção parcial do folheto, colocação de anel tricúspide e recolocação do electrocatéter de pacing no ventrículo direito.
Observou-se regressão dos sintomas de congestão, objetivando-se o anel protésico tricúspide sem evidência de estenose e cavidades direitas apenas ligeiramente dilatadas, com hipertensão pulmonar moderada. Cerca de seis meses após o procedimento, a doente sofreu um evento neurológico agudo, com evolução desfavorável, tendo vindo a falecer.
Tricuspid regurgitation (TR) has tended to receive less attention than aortic and mitral valve disease, and the tricuspid is often referred to as the ‘forgotten valve’.1
According to the Framingham Heart Study, the prevalence of moderate or severe TR is approximately 0.8% and increases with age. It is four times more common in women.2 TR is normally associated with mitral valve disease; more than a third of patients with mitral stenosis suffer from at least moderate TR.3
The etiology of TR can be primary (intrinsic) or secondary (functional). Primary TR results from structural changes to the valve apparatus and can be congenital or acquired; it accounts for only 8–10% of cases of severe regurgitation.4 Secondary TR is normally due to tricuspid annulus dilatation following right ventricular (RV) dilatation and dysfunction, which may be primary or secondary to left-sided heart disease causing pulmonary hypertension. In most patients, TR is functional and unrelated to primary heart valve disease. It is often observed in advanced stages of left-sided heart valve disease and myocardial disease. In 14% of patients, TR occurs in the absence of structural changes in the valve, pulmonary hypertension or left heart failure.5
Risk factors for persistent and worsening TR include atrial fibrillation, the presence of pacemaker leads, pulmonary hypertension, RV remodeling and increased RV pressure, and the severity of the valve defect.6 In cases of significant regurgitation, progressive RV remodeling and dysfunction due to chronic volume overload result in the detachment of the papillary muscles and valve leaflet separation, leading to worsening regurgitation and further RV dilatation. Moreover, the dilated right ventricle may compress the left ventricle, leading to an increase in pulmonary pressure and worsening of TR. The presence of a flail leaflet, which is known to be linked to severe regurgitation, is associated with worse survival and greater risk of heart failure.7
Morbidity and mortality in tricuspid valve surgery are higher than in most heart surgery procedures, and decisions regarding surgery and its timing are therefore dependent on the severity of regurgitation, RV function, the dimensions of the tricuspid annulus and clinical manifestations, as well as the presence of pulmonary hypertension and atrial fibrillation.8
Case reportWe present the case of a 74-year-old woman with a history of hypertensive heart disease with preserved global systolic function, dyslipidemia and permanent atrial fibrillation, anticoagulated with rivaroxaban and also taking amlodipine and bisoprolol. She had a DDDR pacemaker implanted in 2005 due to symptomatic complete atrioventricular block, with no reported complications since placement, and was followed regularly at the cardiology department. She underwent a transthoracic echocardiogram in 2009 which showed evidence of fibrocalcific mitral valve disease with mild mitral regurgitation and no other significant findings.
In 2011, the patient developed fatigue on moderate exertion and abdominal discomfort, without dyspnea or orthopnea. Laboratory testing revealed worsening hepatic cholestasis, prompting referral for gastroenterology and internal medicine consultations. The cholestasis was initially attributed to the use of calcium channel blockers, which were discontinued, without improvement. In 2012, a new episode of symptomatic complete atrioventricular block due to battery depletion was observed, and the pacemaker generator was replaced without manipulating the existing leads. At this time, the patient underwent a repeat echocardiogram which revealed septal dyskinesia secondary to pacing, and moderate tricuspid regurgitation with mild pulmonary hypertension. The pacemaker lead was visualized in the right chambers. Twenty-four-hour Holter monitoring performed in 2013 confirmed that sensing function was maintained with predominantly pacing rhythm (93% of the recording). The patient continued to experience progressive worsening of generalized fatigue and weakness, with the onset of ascites, abdominal pain and insidious weight loss, accompanied by lower limb edema, with no evidence of fever or dyspnea, and she sought medical advice again in 2015.
Etiological investigation at this time included abdominal ultrasound, which revealed homogeneous hepatomegaly and dilatation of the inferior vena cava and upper hepatic veins, suggestive of congestive hepatopathy, while echocardiography showed severe tricuspid regurgitation (proximal isovelocity surface area radius >12mm), which had worsened compared with serial exams performed over the previous four years, with RV dilatation and dysfunction (tricuspid annular plane systolic excursion [TAPSE] 13mm). There was no significant pulmonary hypertension and left ventricular function was still preserved. The transthoracic echocardiogram was therefore repeated, revealing a d-shaped left ventricle with marked dilatation of the tricuspid valve annulus, and also valve thickening, without coaptation of the tricuspid leaflets due to the flail septal leaflet. This appeared to be caused by the tension exerted by the RV pacemaker lead, which formed a loop over the interatrial septum, causing severe TR (regurgitant orifice area of 15mm with flow characterized as “unmeasurable”) (Videos S1 and S2 show preoperative three-dimensional, two-dimensional and color Doppler transthoracic echocardiographic images of mechanical interference of the pacemaker lead in the tricuspid valve with severe regurgitation).
Given the patient’s poor prognosis, we opted for minimally invasive surgery via a right thoracotomy. Perforation of the tricuspid septal valve leaflet by the pacemaker lead was confirmed intraoperatively. The lead was removed and repositioned by the anteroseptal commissure of the tricuspid valve, followed by partial resection of the leaflet and closure of the orifice with 4-0 polypropylene sutures and placement of a 32mm Carpentier-Edwards ring. No complications arose during the procedure. However, in the postoperative phase, due to sporadic capture failure requiring progressive increases in the sensing threshold to unacceptable values, and given that the patient was completely RV pacing-dependent, it was decided to upgrade the DDDR pacemaker to a cardiac resynchronization therapy pacemaker, placing the left ventricular lead anterior to the coronary sinus.
The patient’s postoperative course was favorable with regression of clinical symptoms of congestion and increased tolerance of moderate and strenuous exertion. Echocardiographic follow-up revealed no evidence of stenosis in the prosthetic tricuspid ring and the tension previously exerted by the lead was not observed. TR was recorded as mild (vena contracta 5mm) and the right chambers were also only slightly dilated (TAPSE 16mm), with moderate pulmonary hypertension (pulmonary artery systolic pressure 40mmHg). Videos S3 and S4 show postoperative two-dimensional and color Doppler transthoracic echocardiography revealing mild TR.
The patient was followed regularly at the cardiology department, remaining clinically stable and reporting no symptoms that affected her quality of life.
However, six months after the procedure she suffered a vertebrobasilar ischemic stroke with extensive hemorrhagic transformation, and deteriorated and died during hospitalization.
DiscussionAlthough mild TR is detected in 80–90%2 of healthy individuals on echocardiography and is normally benign, hemodynamically significant regurgitation can cause debilitating symptoms and is associated with poor prognosis in many cardiovascular diseases. It is therefore important to understand its pathophysiology and to assess its severity, and patients need to be referred in a timely manner to prevent clinical deterioration and associated complications.
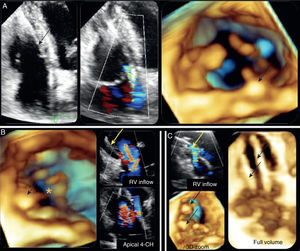
TR following endocardial lead placement or removal of a pacemaker or implantable cardioverter-defibrillator is a known complication of these procedures. Data relating to its prevalence are conflicting and the reported incidence ranges from 7% to 39%.9 The mechanisms involved include mechanical interference of the leads in leaflet closure (through leaflet perforation, adherence to the valve or entanglement in the subvalvular apparatus), as well as pacing-induced RV dyssynchrony, leading to valve dysfunction and hence regurgitation.9 In an autopsy study of 26 patients with pacemakers, Sakai et al. reported mechanical interference in the valve leaflets in 42% of cases10 (Figure 1).
(A) Two-dimensional (left) and three-dimensional (3D) (right) apical 4-CHamber (4-CH) views showing pacemaker lead interference in the septal leaflet with tricuspid regurgitation; (B) 3D view from the right ventricle (RV) showing absence of coaptation of the valve leaflets and severe regurgitation with leads in situ; (C) 3D view from the RV revealing two pacemaker leads through the tricuspid valve with moderate regurgitation.11
The time taken for TR to develop and progress following placement of pacemaker leads has not been studied in depth. Pathological studies have shown significant cardiac inflammation days after placement.12 Progressive inflammation over weeks and months can lead to the formation of fibrous tissue around the leads, resulting in strong adherence to various parts of the tricuspid valve apparatus, and leading to regurgitation. Prompt detection of this complication is paramount as it can only be resolved by early lead replacement, a procedure that can be difficult, if not impossible, to perform at an advanced stage.
According to the European Society of Cardiology guidelines on valvular heart disease, surgery is indicated in patients with severe tricuspid regurgitation undergoing left-sided valve intervention (class of recommendation I, level of evidence C) and should be considered in mild or moderate regurgitation with significant tricuspid annulus dilatation (≥40mm or >21mm/m2) (class IIa, level C). Isolated tricuspid valve surgery should however be considered in symptomatic patients with severe regurgitation and RV dysfunction (class IIa, level C). Ring annuloplasty is key to surgery for secondary TR. In the presence of transtricuspid pacemaker leads, the technique used should be adapted to the patient’s condition and the surgeon’s experience.
In the case presented, given the patient’s severe TR with significant annulus dilatation and severe right-sided heart dysfunction, surgery to remove the pacemaker lead and annuloplasty were required. Assuming that the lead could be repositioned in the right ventricle under direct visualization, thereby resolving the regurgitation, we did not opt for epicardial pacing as this would have involved tunneling the lead to a pectoral position, with increased risk of fracture. As it was not known whether coronary sinus catheterization would be possible, left pacing using this method was not initially considered as the first option. The presence of pulmonary hypertension in this patient, with no left-sided valve disease and with good left ventricular systolic function, remains unexplained, although the fact that it worsened was likely a consequence of two consecutive cardiac procedures.
Ten-year postoperative survival in tricuspid valve repair is 30–50%. Major predictive factors are preoperative functional class, right and left ventricular function, and prosthetic complications.13 In this case, there was a four-year interval between the onset of the first symptoms and diagnosis, which allowed the disease to progress, leading to the development of RV dilatation and dysfunction and significant constitutional symptoms. Despite this, the postoperative outcome was satisfactory, the patient having regained good functional status and autonomy.
In summary, this case illustrates the risk of severe and potentially irreversible complications arising from routine procedures. The non-specific nature of the symptoms highlights the importance of maintaining a high index of suspicion with regard to this rare complication. Regular clinical follow-up and serial echocardiography are required to assess its impact.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Toscano M, Neves Z, Matias C, Carvalho M, Ribeiras R, Morgado F, et al. Electrocatéter como causa iatrogénica de insuficiência cardíaca direita – relato de um caso. Rev Port Cardiol. 2019;38:675–676.