Diagnosis of Brugada syndrome (BS) currently requires documentation of a characteristic repolarization pattern (type 1 Brugada ECG). Mutations in the SCN5A gene, which codes for sodium channel NaV 1.5, are found in 38% of familial cases of BS. Sodium current dysfunction negatively affects the cardiac fast response action potential, particularly in atrial and ventricular myocytes and in the fast-conducting Purkinje system.
ObjectivesTo detect carriers of SCN5A mutations without using the characteristic repolarization pattern (type 1 Brugada ECG).
MethodsOf a total of 141 members of three different families including 55 carriers of two nonsense SCN5A mutations causing BS, all those aged over 16 (113 individuals, 42 carriers) were studied. The PR interval (PR) and QT dispersion (QTd) between leads V1 and V3 were measured on conventional ECG. Using signal-averaged ECG the total duration of the filtered QRS complex (fQRS), the root-mean-square (RMS40) and the low-amplitude signal (LAS) were measured. The following procedures were developed to detect carriers: (1) a screening test (ScreenTest) with PS (PR+fQRS)≥250 (250ms is 80% of the theoretical maximum in healthy individuals); and (2) a diagnostic test (DiagTest) for the simultaneous fulfillment of four conditions: PS≥250 and QTd≥10 and LAS>26 and RMS40 ≤29 (the latter two cut-offs are approximately 70% of the theoretical maximum in healthy carriers).
ResultsSignificant differences in PR, QTd, fQRS, RMS40 and LAS were found between carriers and non-carriers. The SCN5A gene was associated with all these variables, the strongest association being with PR. Both tests were applied to 63 family members (38 carriers). The ScreenTest was positive in 38 of 38 carriers, with eight false positives in 27 non-carriers (sensitivity [SE]=100% and specificity [SP]=66.67%). From ROC curve analysis a cut-off of PS=252.5 shows SE=100% and SP=76% and a cut-off of PS=260 shows SE=94.7% and SP=84%. The DiagTest was positive in 36 of 38 carriers, with three false positives: SE=94.74% and SP=88.89%. From ROC curve analysis a multivariate logistic model identifies a cut-off with SE=92% and SP=92%. In the same group the SE and SP of the characteristic spontaneous repolarization pattern (type 1 Brugada ECG) to detect carriers were 52.4% and 97.2%, respectively, and the difference between the SE of the DiagTest and of the typical repolarization pattern is statistically significant.
ConclusionsThe ScreenTest and DiagTest are more effective tools than the characteristic repolarization pattern to discriminate between carriers and non-carriers of these two nonsense SCN5A mutations. We suggest their use in first-degree relatives of Brugada patients when the results of genetic testing are not available, in a score of disease probability in individuals with idiopathic Brugada ECG, and in patients with arrhythmias or other Brugada-related symptoms presenting type 2 or type 3 Brugada ECG.
Atualmente, o diagnóstico da Síndrome de Brugada (SB) obriga à documentação do padrão eletrocardiográfico de repolarização característico denominado tipo 1. Em 38% dos casos familiares desta entidade encontra-se uma mutação do gene SCN5A responsável pela síntese do canal de sódio NaV1.5. A disfunção desta corrente de sódio repercute-se no potencial de ação cardíaco de resposta rápida, fundamentalmente nos miócitos auriculares, nas fibras de Purkinje e nos miócitos ventriculares.
ObjetivosDetetar portadores de mutação do SCN5A sem recorrer ao padrão de repolarização no ECG.
MétodosA partir de 3 famílias e de um total de 141 elementos, dos quais 55 portadores (P+) de duas mutações non-sense específicas do SCN5A causadoras de SB, foram estudados os maiores de 16 anos (113 elementos/42 P+). Foi medido no ECG o intervalo PR (PR) em DII e dispersão QT (dQT) entre V1 e V3. No ECG de alta resolução (ECGAR) determinado o QRS filtrado (QRSf), root-mean square (RMS40) e low-averaged signal (LAS). Para detetar P+ foi criado: 1) um teste de rastreio (TesteRas) constituído pelo intervalo PS (PR+QRSf)≥250 (250ms corresponde a 80% do valor teórico máximo deste intervalo em saudáveis) e 2) um teste diagnóstico (TesteDx) que resulta do cumprimento simultâneo de 4 condições: PS≥250 e dQT>10 e LAS≥26 e RMS40≤29 (estes 2 últimos valores correspondem aproximadamente a 70% do valor teórico máximo em saudáveis).
ResultadosEncontradas diferenças significativas entre os P+ versus não portadores (P-) do PR, da dQT, do QRSf, do LAS e da RMS40. O gene está associado a todas estas variáveis, sendo a associação mais forte com o PR. A 63 elementos (38 P+) foi aplicado o TesteRas e o TesteDx. O TesteRas foi positivo em 38 de 38 P+ com 8 falsos positivos em 27 P- (sensibilidade [S]=100% e especificidade [E]=66,67%). Na curva Roc para este teste o cut-off PS=252,5 tem S=100% e E=76% e o cut-off PS=260 tem S=94,7% e E=84%. O TesteDx foi positivo em 36 de 38 P+ com 3 falsos positivos: S=94,74% e E=88,89%. Por modelo logístico multivariado identifica-se um cut-off através da curva Roc com S=92% e E=92%. A S e E de diagnóstico do ECG espontâneo usando o padrão de repolarização tipo 1 no mesmo grupo de doentes foram de 52,4% e 97,2%, respetivamente (a diferença de S entre o TesteDx e o padrão de repolarização no ECG é estatisticamente significativa).
ConclusõesO TesteRas e o TesteDx são uma ferramenta mais eficaz do que o padrão de repolarização típico para distinguir entre P+ e P- destas duas mutações non-sense do SCN5A. Sugerimos a sua utilização em familiares diretos de casos índice com SB, quando o resultado genético (ainda) não está disponível e num score de probabilidade de doença em indivíduos com ECG de Brugada idiopático ou em indivíduos com sintomas relacionados com arritmias e padrão de repolarização tipo 2 ou 3 de Brugada.
Brugada syndrome (BS) was first described as a new entity in 1992. It has a characteristic electrocardiographic pattern (right bundle branch block and ST-segment elevation in the right precordial leads) and is associated with increased risk for malignant ventricular arrhythmias and sudden death in individuals without structural heart disease.1 In two decades it has ceased to be a medical curiosity and has become an entity that must be diagnosed, stratified and treated, since it is estimated to be responsible for 20–50% of cases of sudden death in individuals with structurally normal hearts,2 and the mean age at diagnosis and/or the event that prompts it (which is often sudden death) is 40±22 years. BS belongs to the group of channelopathies, diseases that are caused by primary dysfunction of the ion channels responsible for cardiac action potentials, which triggers or perpetuates arrhythmias without concomitant structural disease.3
Historical perspectiveBS is a congenital disease and familial transmission is frequent. The first mutation associated with BS, in the SCN5A gene which codes for the α subunit of the cardiac sodium channel NaV 1.5,4 was described in 1998, and since then over 100 different mutations in this gene have been reported.5 These mutations may be nonsense or missense, and not all have the same pathophysiological effects. The former are usually more serious, as they produce a truncated protein that is dysfunctional because it is a different length than normal. Missense mutations cause substitution of an amino acid but the protein remains complete. In this case the biological effects on protein function are unclear, and in vitro functional studies are needed to ascertain their importance.
Mutations in the SCN5A gene can lead to a decrease in sodium current (INa) in the cardiac action potential, either through quantitative decrease (failure in expression) or through qualitative dysfunction of the channel. However, mutations are found in only 18–30% of patients2 and in only 38% of familial cases.6 This low incidence may be explained by genetic heterogeneity, i.e. mutations in other genes that are associated with BS, as well as many others presumably yet to be discovered, including: (1) GPD1-L, the gene that codes for glycerol-3-phosphate dehydrogenase 1 like peptide, which is responsible for the transport of NaV1.5 to the cell surface and, when mutated, leads to a 31% reduction in the quantity of channels available in the membrane and a 50% reduction in sodium current, resulting in BS7; (2) mutations in cardiac calcium channel genes (CACNA1c and CACNB2b) associated with an overlapping entity, short QT syndrome with Brugada ECG pattern8; and (3) mutations in the KCNE3 gene, which modulates the function of the KV4.3 channel, which is responsible for the transient potassium outward current (ITO).9 Apart from those recently described in the SCN5A gene, all these mutations have made it necessary to widen the original idea of BS as a “pure” sodium channelopathy to the currently accepted concept of it as a channelopathy of phase 1 of the cardiac action potential due to an imbalance in one of the different ion currents (sodium, potassium or calcium), caused by mutations in either ion channel genes or in genes coding for proteins associated with the activity or transport of these currents.10
Diagnosis of BSDiagnosis of BS is currently only considered definitive when a type 1 Brugada ECG pattern is observed in at least two right precordial leads, in the presence or absence of a sodium-channel blocking agent, in conjunction with one or more of the following: documented ventricular fibrillation (VF), documented ventricular tachycardia (VT), a family history of sudden cardiac death at <45 years old, type 1 ECG in family members, inducibility of VF/VT with programmed electrical stimulation in electrophysiological study, syncope, or nocturnal agonal respiration.2
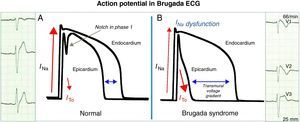
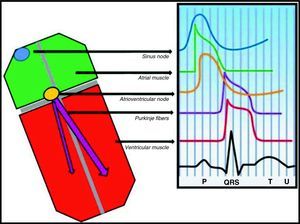
From gene to electrocardiogramThere are two theories to explain the electrocardiographic manifestations of BS: (1) delayed conduction in the free wall epicardium of the right ventricular outflow tract; (2) premature repolarization of the right ventricular epicardial action potential; or a combination of the two. In the first, asynchronous activation of the epicardium in relation to the endocardium, due to dysfunction of an ion channel (NaV1.5 or another), creates a voltage gradient between the two layers of cells. In the second (Fig. 1), dysfunction of a channel (NaV1.5 or another) leads to an imbalance in phase 1 that favors repolarization due to the disproportionate ITO current (positive outward current), with the result that the membrane repolarizes beyond the voltage at which L-type calcium channels are activated and phase 2 of the action potential thus fails. In both models a transmural voltage gradient is generated between the epicardium and the endocardium that appears on the ECG as a coved ST-segment elevation of ≥2mm (0.2mV), followed by a negative T wave (type 1 Brugada ECG) that acts as an arrhythmic substrate for phase 2 reentry. Both hypotheses explain the preferential involvement of the right ventricular outflow tract since the ITO current has greater functional expression in the epicardium of this region. The repolarization alterations on the ECG that are diagnostic of BS reflect dysfunction of the ventricular fast response action potential, which is also responsible for depolarization of atrial myocytes and Purkinje fibers (Fig. 2).11,12 It can therefore be hypothesized that the action potential dysfunction responsible for the repolarization disturbances described above may also lead to increases, or even subclinical or clinical disturbances, in intra-atrial, atrioventricular and intraventricular conduction.
A: In healthy individuals, there is a small notch in phase 1 of the ventricular action potential mediated by the ITO current that is more evident in the epicardium and is not visible on the ECG. B: In Brugada syndrome, an ionic imbalance in phase 1 favoring repolarization in the epicardial surface generates a transmural voltage gradient, seen on the ECG as type 1 repolarization pattern.
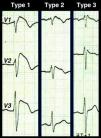
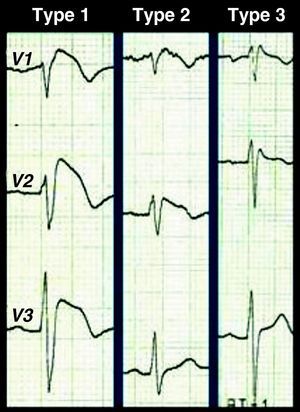
A diagnosis of BS is simple in its most characteristic form of presentation, i.e. spontaneous type 1 repolarization pattern in the right precordial leads together with aborted cardiac arrest. Type 1 repolarization pattern with no other criteria should be described as idiopathic Brugada ECG (not Brugada syndrome), and there is considerable controversy concerning the appropriate clinical approach. A wide range of differential diagnoses that can mimic the BS ECG alterations should be considered (Table 1),5 but it should be borne in mind that the first symptom that confirms a diagnosis of BS may be catastrophic: cardiac arrest is the first manifestation in 77% of patients. There are also difficulties with diagnosis in cases of borderline or Brugada-like repolarization patterns. Besides type 1 Brugada ECG, there are two other repolarization patterns associated with BS (Fig. 3): type 2 (≥2mm J-point elevation, downsloping ST segment but at least 1mm above the isoelectric line, and positive or biphasic (‘saddle-back’) T wave; and type 3, with ≥2mm J-point elevation and <1mm ST-segment elevation, coved or saddle-back or both. According to the consensus conference on Brugada syndrome, types 2 and 3 are merely suggestive and not diagnostic, a recommendation that is not followed by all authors.13,14 Whether only type 1, or all three types of pattern, are taken to be diagnostic obviously affects the sensitivity and specificity of the ECG to detect BS, sensitivity being greater when all three are used and specificity being higher with only type 1. Type 3 pattern is virtually indistinguishable from the incomplete right bundle branch block found in the young; disregarding it could have catastrophic results, but an incorrect diagnosis of BS would place an enormous burden on a healthy individual and their family, with serious consequences for their personal, social, and professional life. This dilemma has aroused controversy in the literature and is presumed to have “contaminated” some databases with healthy individuals, which would explain the significant differences in reported prognosis for this entity.15–17
Clinical situations that can mimic the Brugada repolarization pattern.
| Atypical right bundle branch block |
| Right ventricular myocardial infarction |
| Acute pericarditis |
| Hemopericardium |
| Pulmonary thromboembolism |
| Aortic dissection |
| Central nervous system disorders |
| Duchenne muscular dystrophy |
| Friedreich's ataxia |
| Left ventricular hypertrophy |
| Arrhythmogenic right ventricular cardiomyopathy |
| Mechanical compression of the right ventricular outflow tract (tumor/pectus excavatum) |
| Following electrical cardioversion |
| Early repolarization |
| Hypothermia |
When diagnosis is confirmed in any index case, all family members who might be affected should be tested with baseline ECG and/or provocation testing. Baseline ECG alone is insufficient, since 51% of affected individuals fluctuate between diagnostic and non-diagnostic ECGs.18 Provocation testing with sodium channel blockers such as flecainide, ajmaline or procainamide is thus essential to detect intermittent or occult forms of the disease, although little is known of their sensitivity, specificity and reproducibility.19
Signal-averaged ECGSignal-averaged ECG (SAECG) is a computer-based technique that is able to detect QRS abnormalities that are too subtle for conventional ECG. This exam (Fig. 4) shows the arithmetic mean of the sum of multiple (usually 200) QRS complexes, obtained over a period of approximately five minutes with as little background noise as possible. The process increases the signal-to-noise ratio of the selected cardiac complexes so that signals of the order of one microvolt in the terminal QRS complex can be detected and measured to within a few milliseconds. These signals are commonly known as ventricular late potentials.20
ObjectivesThe aims of the study were to construct a model to detect carriers of nonsense or missense SCN5A mutations with significant functional repercussions based on subclinical disturbances in intra-atrial, atrioventricular and intraventricular conduction. This is used to develop a score of disease probability which we suggest should be used in first-degree relatives of Brugada patients and in individuals with idiopathic Brugada ECG.
MethodsOf a total of 141 members of three different families including 55 carriers of two nonsense SCN5A mutations causing BS (43 with Arg222STOP and 12 with Thr1754ProfsX32), all those aged over 16 were studied.
The PR interval (PR) in DII and QT dispersion (QTd) between leads V1 and V3 were measured on conventional ECG, calibrated so that 20mm=1mV, and with a velocity of 50mm/s. Signal-averaged ECG was performed with time-domain analysis, and the total duration of the filtered QRS complex (fQRS), the root-mean-square (RMS40) and the low-amplitude signal (LAS) were measured. Two tests were developed to detect carriers:
(1) a screening test (ScreenTest) with PS (the sum of PR and fQRS)≥250 (the theoretical maximum of this interval in healthy individuals is 314ms [200+114], 250ms being 80% of this figure);
(2) a diagnostic test (DiagTest) for the simultaneous fulfillment of four conditions: PS≥250 and QTd>10 and LAS>26 and RMS40≤29 (the latter two cut-offs are approximately 70% of the theoretical maximum in healthy carriers).
ResultsThe study population consisted of 113 individuals, 42 of them carriers. Significant differences in PR, QTd, fQRS, RMS40 and LAS were found between carriers and non-carriers. The SCN5A gene was associated with all these variables, the strongest association being with PR (Table 2). Both tests were applied to 63 family members (38 carriers). The ScreenTest was positive in 38 of 38 carriers, with eight false positives in 27 non-carriers (sensitivity=100% and specificity=66.67%). From ROC curve analysis (Fig. 5) a cut-off of PS=252.5 shows sensitivity=100% and specificity=76% and a cut-off of PS=260 shows sensitivity=94.7% and specificity=84%. The DiagTest was positive in 36 of 38 carriers, with three false positives: sensitivity=94.74% and specificity=88.89%. From ROC curve analysis a multivariate logistic model identifies a cut-off with sensitivity=92% and specificity=92%. The area under the curve was 0.927 (p<0.001), demonstrating that this test has an excellent ability to discriminate between carriers and non-carriers.21 In the same group the sensitivity and specificity of the characteristic spontaneous repolarization pattern (type 1 Brugada ECG) to detect carriers were 52.4% and 97.2%, respectively, and the difference between the sensitivity of the DiagTest and of the typical repolarization pattern is statistically significant.
Clinical, demographic and electrical characteristics of the study population.
| Carriers | Non-carriers | p | Association with SCN5A mutation (eta coefficient) | |
| Clinical and demographic characteristics | ||||
| Male – n (%) | 20 (47.6) | 34 (47.9) | 0.567 | – |
| Age (years) | 40.31±14.81 | 35.08±13.05 | 0.059 | – |
| Spontaneous type 1 ECGa – n (%) | 22 (52.4) | 2 (2.8) | <0.001 | – |
| Spontaneous type 2/3 ECGb – n (%) | 5 (11.9) | 4 (5.6) | 0.490 | – |
| Provocation test – n total/n positive | 17/8 | 10/2 | 0.161 | – |
| Symptoms – n (%) | 6 (14.3) | 4 (5.6) | 0.112 | – |
| EPS – n total/n positive | 18/3 | 5/0 | 0.461 | – |
| ICD – n (%) | 8 (25.8) | – | – | – |
| ECG and SAECG | ||||
| PR | 190.98±27.09 | 151.37±22.18 | <0.001 | 0.653 |
| QTd | 47.14±23.51 | 28.95±18.04 | <0.001 | 0.472 |
| fQRS | 106.13±9.40 | 92.41±10.76 | <0.001 | 0.544 |
| LAS | 41.92±9.75 | 31.59±11.82 | <0.001 | 0.401 |
| RMS40 | 18±6.55 | 35.26±21.8 | <0.001 | 0.47 |
ICD: implantable cardioverter-defibrillator; LAS: low-amplitude signal; PS: PR interval+filtered QRS complex; QTd: QT dispersion; RMS40: root-mean-square; SAECG: signal-averaged ECG.
In 2008 it was demonstrated that carriers of truncating (nonsense) SCN5A mutations have more symptomatic forms of BS, and atrioventricular conduction disturbances were also described, both through increased PR interval22,23 and increased HV interval measured invasively during electrophysiological study.24 SAECG was proposed around the same time as a possible tool for arrhythmic risk stratification in BS.25
However, this study is the first to propose incorporating conduction parameters into the diagnostic process. As with long QT syndrome26 and arrhythmogenic right ventricular cardiomyopathy,27 the proposed test could be included in a score of disease probability (Table 3), which we consider would be useful given the shortcomings discussed above of using patterns of ventricular repolarization in isolation and the clinical and social burdens arising from a diagnosis of BS.
Diagnostic criteria of Brugada syndrome.
| Criterion | Points |
| ECG | |
| Type 1 ECG | 5 |
| Type 2/3 ECG | 4 |
| PS≥250 and QTd>10 and LAS≥26 and RMS40≤29 | 2 |
| Type 1 ECG in relative(s) | 3 |
| Documented VF | 3 |
| Documented VT | 3 |
| VF/VT inducible by programmed electrical stimulation | 3 |
| Clinical features | |
| Family history of sudden cardiac death at <45 years old | 3 |
| Syncope | 3 |
| Nocturnal agonal respiration | 3 |
| Probability of BS | |
| ≥8 points: confirmed | |
| 7 points: highly probable | |
| 6 points: possible | |
| ≤5 points: unlikely | |
BS: Brugada syndrome; LAS: low-amplitude signal; PS: PR interval+filtered QRS complex; QTd: QT dispersion; RMS40: root-mean-square; VF: ventricular fibrillation; VT: ventricular tachycardia.
When there is clinical and/or electrocardiographic suspicion of BS, intra-atrial, atrioventricular and intraventricular conduction times should be measured, ideally with SAECG, since the fast response cardiac action potential that is dysfunctional in BS is involved in all three. Simultaneous detection of times above the normal-high limit of these three depolarization times (in this study the limit used was 70–80% of the theoretical maximum in healthy individuals) may indicate the likelihood of the individual having the disease due to nonsense or missense mutations in SCN5A with significant functional impact. We suggest their use in adult first-degree relatives of index cases when the results of genetic testing are not available. Another possible use would be to calculate the probability of disease in individuals with idiopathic Brugada ECG or with arrhythmias and type 2 or 3 Brugada repolarization pattern. In these cases, the proposed score could support the diagnosis (score 7), raise suspicion (score 6), or render it unlikely (score ≤5).
ConclusionWe have shown that the ScreenTest and DiagTest are more effective tools than the characteristic Brugada repolarization pattern to discriminate between carriers and non-carriers of nonsense SCN5A mutations.
These tests may be useful in diagnosing this entity by determining the probability that a given repolarization pattern in fact corresponds to BS.
LimitationsOur study has two major limitations:
- 1.
The number of carriers was relatively small and only two mutations were studied. In order to prove the usefulness and reproducibility of the proposed score, it will be necessary to apply it to other Brugada patients and their families.
- 2.
The P wave and the PR interval were not measured with the precision of SAECG, since the means to do so were not available to our group. Measurement of the PS interval, or the PR+fQRS wave, entirely by SAECG (without interference from the atrioventricular node) could make the score even more effective.
We accordingly urge all clinicians working with BS patients who may be interested in collaborating with this work to contact us.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Santos, L; Critérios de diagnóstico da Síndrome de Brugada. Podemos melhorar? Rev Port Cardiol. 2012. doi:10.1016/j.repc.2011.09.023





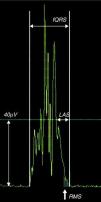




![Late potentials are defined by at least two of three criteria: total filtered QRS duration (fQRS) >114ms, the duration of the terminal QRS complex that is less than 40μV (low amplitude signal [LAS]) >38ms, and the voltage in the last 40ms of the QRS (root-mean square [RMS40]) <20μV.](https://static.elsevier.es/multimedia/21742049/0000003100000005/v1_201305151729/S2174204912000384/v1_201305151729/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)



