Percutaneous septal ablation by alcohol-induced septal branch occlusion was introduced as a new treatment option in symptomatic patients with hypertrophic obstructive cardiomyopathy (HOCM). Our aim was to evaluate procedural and long-term clinical and echocardiographic outcomes in patients with HOCM treated by alcohol septal ablation (ASA) at our center.
MethodsThis single-center retrospective study included 14 consecutive HOCM patients undergoing percutaneous ASA (66.4±12.1 years, 71.4% female). At baseline all patients presented persistent symptoms despite optimized medical treatment, left ventricular outflow tract (LVOT) obstruction with a peak gradient >50mmHg, systolic anterior motion of the mitral valve, and ventricular septal thickness ≥15mm. ASA was considered successful when the LVOT pressure gradient fell to less than 50% of baseline value. All patients had echocardiographic evaluation at baseline, intraprocedure and at follow-up, and a long-term clinical follow-up (25±38 months) with evaluation of functional class and occurrence of symptoms or cardiovascular events.
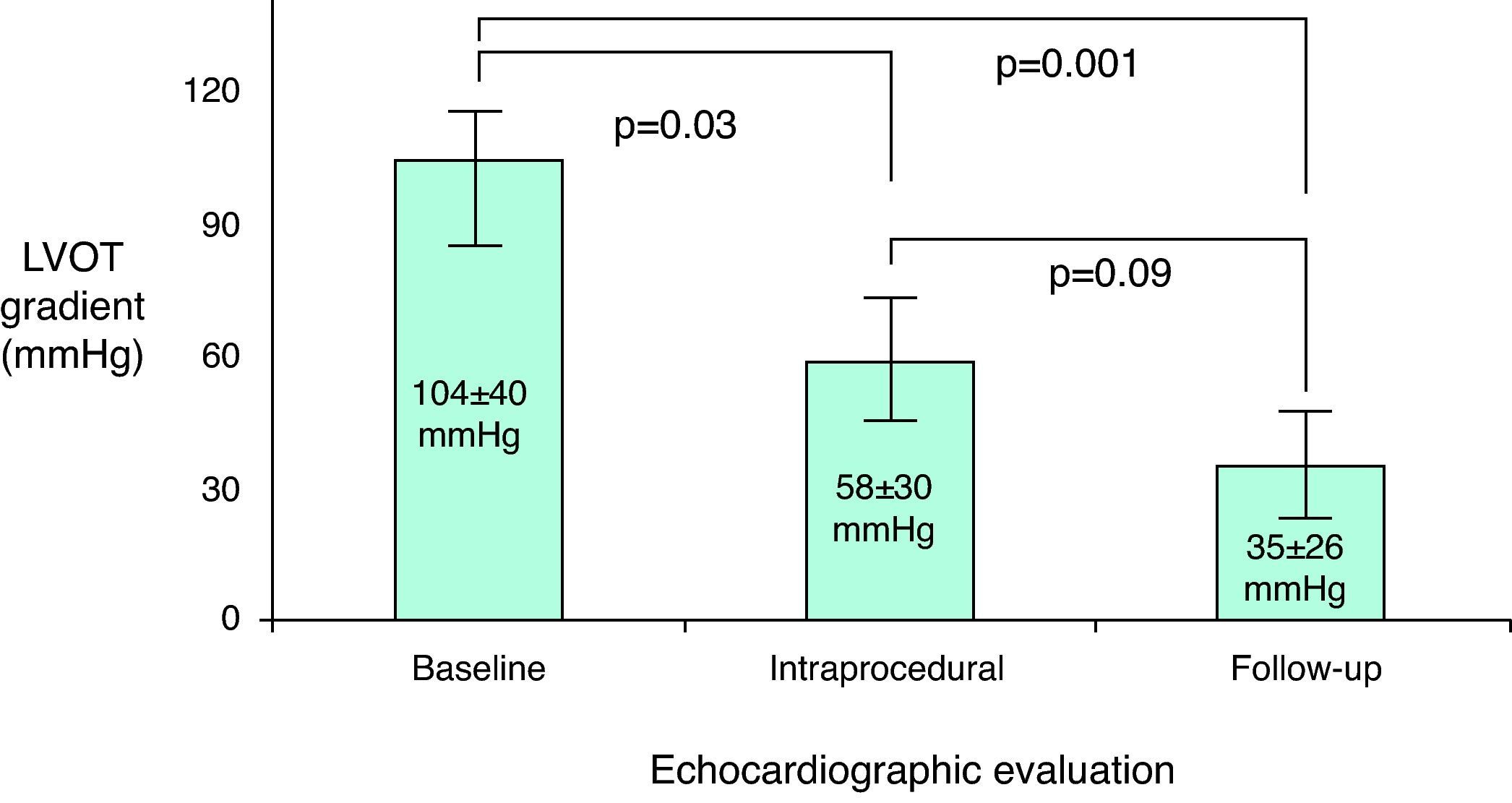
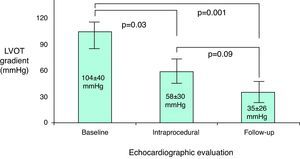
ResultsPercutaneous ASA achieved a 71.4% acute and 85.7% long-term success rate. Peak LVOT gradient decreased from 104±40mmHg at baseline to 58±30mmHg intraprocedure (p=0.03) and 35±26mmHg at follow-up (p=0.001); total gradient decrease was 75±43mmHg. Ventricular septal thickness and mitral regurgitation also presented significant decreases during follow-up (from 24±5mm to 18±4mm, p=0.02, and from grade 2.4±0.6 to 1.4±0.5, p<0.001, respectively). A tendency for long-term improvement in NYHA functional class (from 2.6±1.1 to 1.8±1.4, p=0.09) was observed. Procedural complications occurred in 6.7% of patients; two deaths and one transient ischemic attack occurred in-hospital, but no long-term clinical events were recorded.
ConclusionsPercutaneous ASA is an effective treatment for symptomatic patients with HOCM, obtaining a marked decrease in LVOT pressure gradient and symptomatic improvement. Despite the occurrence of a significant number of procedural complications, the favorable long-term outcomes underline the potential of ASA as a percutaneous alternative to surgical myectomy.
A ablação septal percutânea por indução alcoólica da oclusão de ramos coronários septais foi introduzida como uma nova opção terapêutica em doentes com cardiomiopatia hipertrófica obstrutiva (HOCM) sintomática. O nosso objetivo foi a avaliação dos resultados clínicos e ecocardiográficos agudos e a longo-prazo em doentes com HOCM tratados por ablação septal alcoólica (ASA) no nosso centro.
MétodosEstudo monocêntrico, retrospetivo incluindo 14 doentes consecutivos com HOCM submetidos a ASA percutânea (66,4±12,1 anos, 71,4% sexo feminino). Todos os doentes apresentavam basalmente sintomas persistentes apesar de terapêutica médica otimizada, obstrução da câmara de saída do ventrículo esquerdo (LVOT) com gradiente máximo >50mmHg, movimento sistólico anterior da válvula mitral e espessura do septo interventricular ≥15mm. A ASA foi considerada bem sucedida quando o gradiente do LVOT diminuiu para menos de 50% do valor inicial. Todos os doentes foram submetidos a avaliação clínica e ecocardiográfica basal, intraprocedimento e durante o seguimento, com avaliação da classe funcional e ocorrência de sintomas ou eventos cardiovasculares a longo-prazo (25±38 meses).
ResultadosA ASA percutânea obteve uma taxa de sucesso de 71,4% e 85,7%, respetivamente aguda e a longo-prazo. O gradiente máximo do LVOT diminuiu de 104±40mmHg basais para 58±30mmHg intraprocedimento (p=0,03) e 35±26mmHg a longo-prazo (p=0,001); o decréscimo total do gradiente foi de 75±43mmHg. Também a espessura do septo interventricular e a regurgitação mitral apresentaram uma diminuição significativa durante o seguimento (de 24±5mm para 18±4mm, p=0,02, e de grau 2,4±0,6 para 1,4±0,5, p<0,001, respetivamente). Uma tendência para a melhoria a longo-prazo da classe funcional NYHA (de 2,6±1,1 to 1,8±1,4, p=0,09) foi observada. Complicações do procedimento ocorreram em 6,7% dos doentes; duas mortes e um acidente isquémico transitório ocorreram no hospital, não se registando eventos clínicos a longo-prazo.
ConclusõesA ASA percutânea é uma terapêutica eficaz para doentes sintomáticos com HOCM, obtendo uma acentuada diminuição do gradiente de pressão do LVOT e melhoria sintomática. Apesar da ocorrência de um número significativo de complicações periprocedimento, o favorável prognóstico a longo-prazo sublinha o potencial da ASA como uma alternativa percutânea à miectomia cirúrgica.
Hypertrophic obstructive cardiomyopathy (HOCM), first described in the late 1950s, is now recognized to be a primary autosomal dominant myocardial disorder characterized by a dynamic narrowing of the left ventricular outflow tract (LVOT), secondary to asymmetrical septal hypertrophy and systolic anterior motion of the mitral valve. The consequent reduction in cardiac output is responsible for the typical symptoms of dyspnea, angina pectoris and stress-induced syncope, with increased risk for sudden cardiac death in some patients.1
Treatment of patients with symptomatic HOCM aims to reduce functional disability and the extent of outflow tract obstruction and to improve diastolic filling and survival in a disease with a mortality rate estimated at 3–4% per year.2 Administration of negatively inotropic drugs is the treatment of first choice, and succeeds in improving functional capacity and symptoms in a high percentage of patients. However, 5–10% of patients with marked LVOT obstruction have severe symptoms unresponsive to medical therapy.3 Such patients have traditionally been referred for surgical myectomy of the basal septum, which has been the gold standard treatment for decades. This procedure relieves left ventricular (LV) outflow obstruction, providing long-term symptomatic and outcome improvement in most patients, with a mortality of 1–2% in experienced centers.4
Percutaneous alcohol septal ablation (ASA) has emerged as a novel interventional treatment option during the last decade.5 This technique, in which ethanol is injected into one or more septal perforator branches of the left anterior descending coronary artery, achieves a circumscribed infarction of the area supplied by the occluded septal branch, reducing the hypertrophied interventricular septum with consequent expansion of LVOT area and gradient reduction.6 The acute and long-term efficacy of ASA has been proven, with hemodynamic results resembling those of myectomy.7,8
The aim of the present study was to evaluate the acute and long-term clinical and echocardiographic outcome of patients with symptomatic HOCM treated by percutaneous ASA in our center.
MethodsStudy populationThe study included 14 consecutive patients with HOCM who underwent ASA between 1999 and 2010 at the Interventional Cardiology Unit of Hospital La Paz, Madrid, Spain.
The diagnosis of HOCM was based on typical clinical, electrocardiographic, and echocardiographic features, with ventricular myocardial hypertrophy occurring in the absence of any other cardiac or systemic cause. The magnitude of myocardial hypertrophy was assessed by M-mode and two-dimensional transthoracic echocardiography, using standard techniques.
Eligibility criteria for ASA were: (1) persistent severe symptoms, defined as New York Heart Association (NYHA) class III or IV dyspnea and/or Canadian Cardiovascular Society (CCS) class III or IV angina, despite optimized medical treatment; (2) dynamic LVOT obstruction (peak gradient >50mmHg at rest or provocable) caused by systolic anterior motion of the mitral valve; (3) ventricular septal thickness ≥15 mm; (4) absence of significant intrinsic mitral valve disease; and (5) absence of need for concomitant cardiac surgical procedure (such as bypass grafting or valve replacement). All patients gave written informed consent for the procedure.
Percutaneous alcohol septal ablation procedureASA procedures started by obtaining arterial and venous femoral accesses, using the standard Judkins technique, followed by placement of a 6F temporary transvenous pacemaker lead in the right ventricular apex, a 5F pigtail or multipurpose catheter into the LV, and a 6F or 7F guiding catheter in the ascending aorta. Re-evaluation of the patient's hemodynamics and re-measurement of the intracavitary gradient were then performed and continuously monitored throughout the procedure, through simultaneous pressure measurement in the LV and the aorta. Physiologic provocation of the gradient was assessed with the Valsalva maneuver and checking for the Brockenbrough-Braunwald-Morrow sign by inducing a premature ventricular complex.
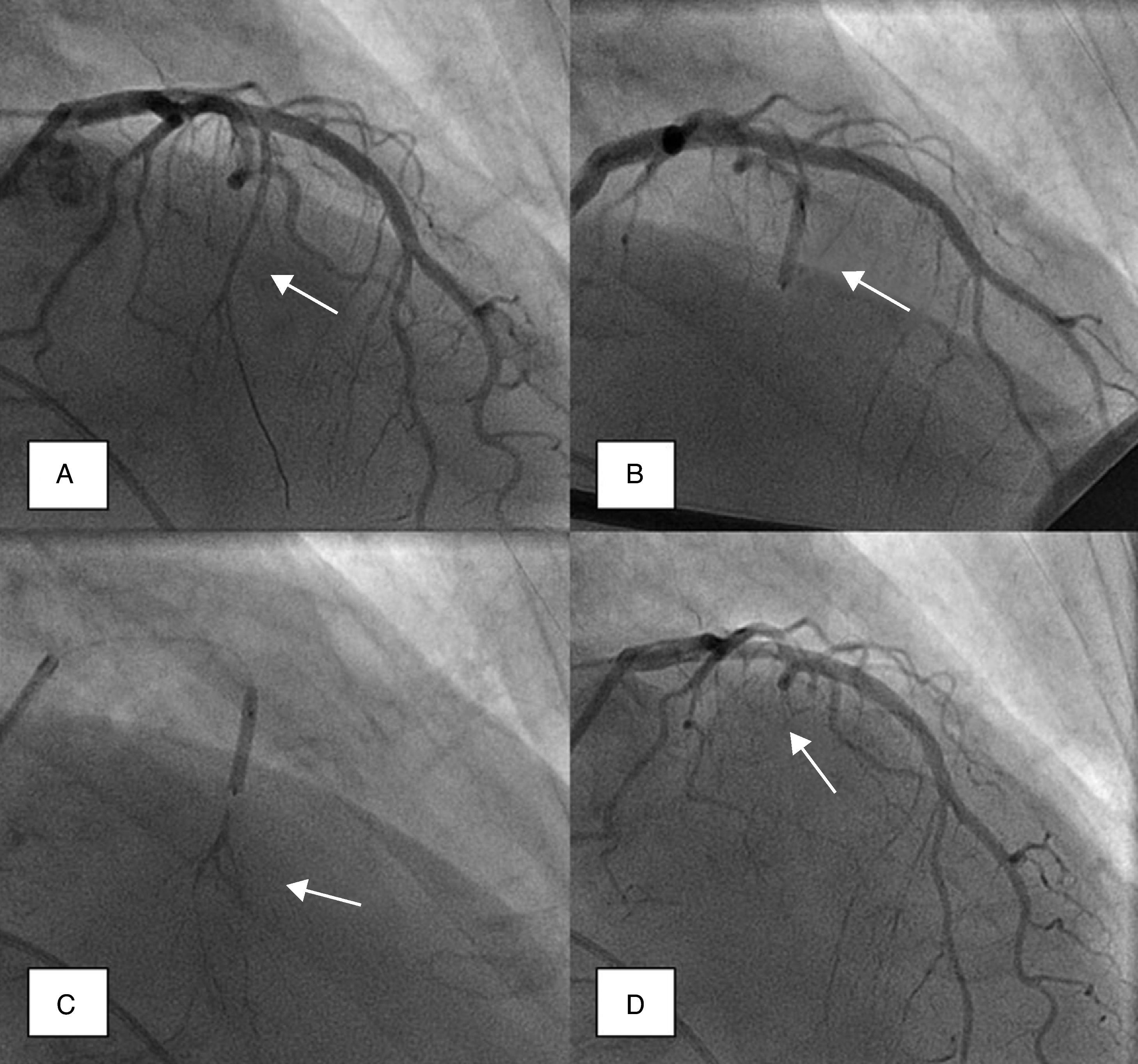
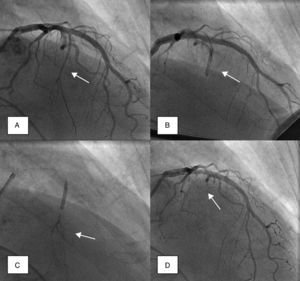
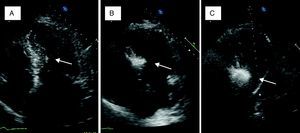
Coronary angiography was then performed to exclude severe coronary disease and to locate the first septal perforator artery. A 0.014-in. guidewire was advanced to engage, in most cases, the first septal branch of the left anterior descending artery (Figure 1A). A slightly oversized, short (∼10mm), over-the-wire angioplasty balloon was then introduced into the septal perforator artery, using standard methods; the lumen of this device provides the route for selective delivery of angiographic contrast, echo contrast, and ultimately alcohol, into the septal artery. After careful fluoroscopic positioning of the balloon (using selective angiography to exclude encroachment onto the LAD), it was inflated and the guidewire removed (Figure 1B).
Angiographic demonstration of alcohol septal ablation technique. Left coronary angiography shows the target septal branch (arrow); an angioplasty guidewire is already inserted inside the septal branch (A). Optimal positioning of the balloon catheter (arrow) in the proximal part of the septal artery without compromise of the left anterior descending artery (B). Injection of angiographic contrast dye through the central lumen of the inflated balloon catheter (arrow) determines the supply area of the septal branch and excludes leakage into the left anterior descending artery or other coronary vessels (C). Final demonstration of the septal artery stump (arrow) after alcohol-induced occlusion (D).
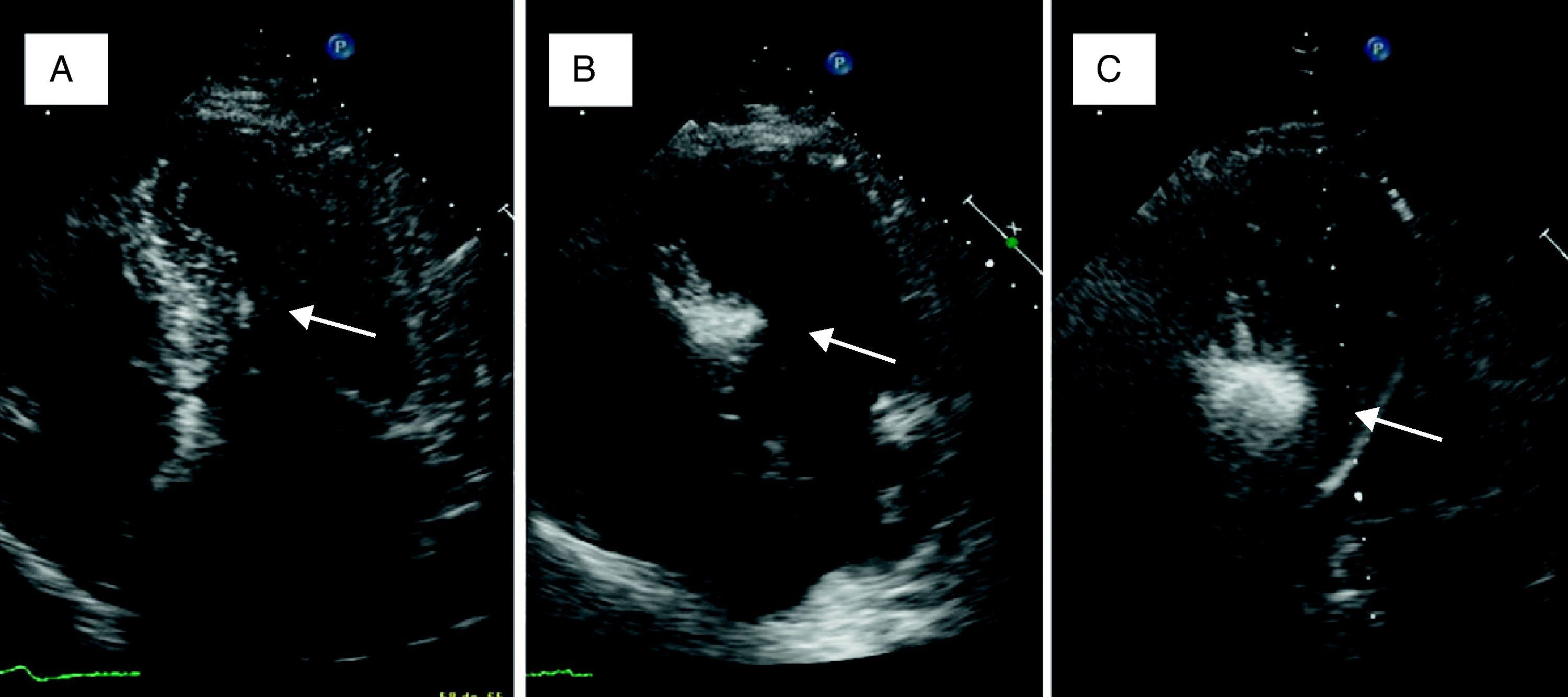
A small amount of angiographic contrast was then injected through the balloon lumen to ensure that there was no spill-back into the LAD or collateral recruitment (Figure 1C). The balloon should not be placed too distally as this may result in a smaller (and solely right-sided) septal infarct, with a consequent reduction in the effect on the outflow gradient. Subsequently, an echocardiographic contrast agent (∼1ml SonoVue, Bracco, Geneva, Switzerland) was injected through the balloon, and the myocardium supplied by the septal artery localized with transthoracic echocardiography (Figure 2). The optimal location within the septum is the point of contact between the anterior mitral valve leaflet and septum in apical four-chamber view. If echocardiographic localization was supportive (no contrast seen outside the thickened basal septum), ablation could proceed. The transvenous pacing wire was re-checked, and intravenous analgesia administered, as the alcohol can cause intense but transient discomfort.
Myocardial contrast echocardiography during alcohol septal ablation. First, the anatomy of the heart is presented and the target septal area is identified (A). Injection of echocardiographic contrast into the target septal branch opacifies the basal part of the septum, verifying the optimal choice of septal branch (B). A more sustained opacification of the basal septum after alcohol injection signifies a good alcohol depot (C).
Absolute alcohol (1–3ml) was then administered slowly through the lumen of the balloon, for 3–5 minutes, followed by saline flush under continuous hemodynamic and ECG surveillance. The invasive and echocardiographic gradients were reassessed, a successful procedure being defined as a residual invasive LVOT pressure gradient of less than 50% of baseline value. If the target reduction in pressure gradient was not achieved, alcohol injection was repeated after 5 minutes (1–2ml) within the same perforator branch. If not successful, the procedure was repeated in a second perforator branch. Once success was achieved, the balloon was deflated, and coronary angiography was repeated to confirm the occlusion of the septal branch and the patency of the left anterior descending coronary artery (Figure 1D). Following deflation, the balloon and wire were removed.
Patients were monitored in an intensive care unit for at least 48 hours after septal ablation, and the temporary pacemaker lead was kept in place for at least 24 hours.
Data collection and follow-upDemographic characteristics, clinical history, echocardiographic and angiographic characteristics, procedural results and in-hospital outcomes were prospectively collected.
Clinical follow-up was obtained in 100% of patients, by in-hospital or telephone interviews with the patient or family and/or review of medical records. Vital status, symptom recurrence, NYHA functional class, need for additional septal reduction therapy (such as surgical myectomy or repeat septal ablation), and possible complications related to septal ablation (such as ventricular arrhythmias or pacemaker dependency) were ascertained by follow-up evaluation. For deceased patients, cause of death was documented from hospital records.
All patients underwent transthoracic echocardiography prior to, during and after the procedure (within the first 24 hours). Left ventricular diameters in end-diastole and end-systole were measured, as well as the thickness of the interventricular septum and left ventricular posterior wall. Left ventricular ejection fraction was accessed by the biplane Simpson method. The peak velocity in the LVOT at rest and after the Valsalva maneuver was measured by continuous wave Doppler echocardiography, and the gradient in the LVOT calculated using the modified Bernoulli equation. Mitral regurgitation was assessed semiquantitatively by color Doppler in four planes. Outpatient echocardiographic follow-up was obtained in 91.7% of patients, by protocol at 3, 6, 12 months and then on a yearly basis. A mean follow-up of 25±38 months was achieved.
Statistical analysisStatistical analysis was performed using dedicated software (SPSS® version 18.0, 2009). Continuous variables were presented as mean values±standard deviation; categorical variables were expressed as frequencies. Comparisons were made using a Student's t-test for paired samples. The Kaplan–Meier method was used to estimate survival curves for the pre-determined end points with 95% confidence intervals. A p value of <0.05 was considered statistically significant.
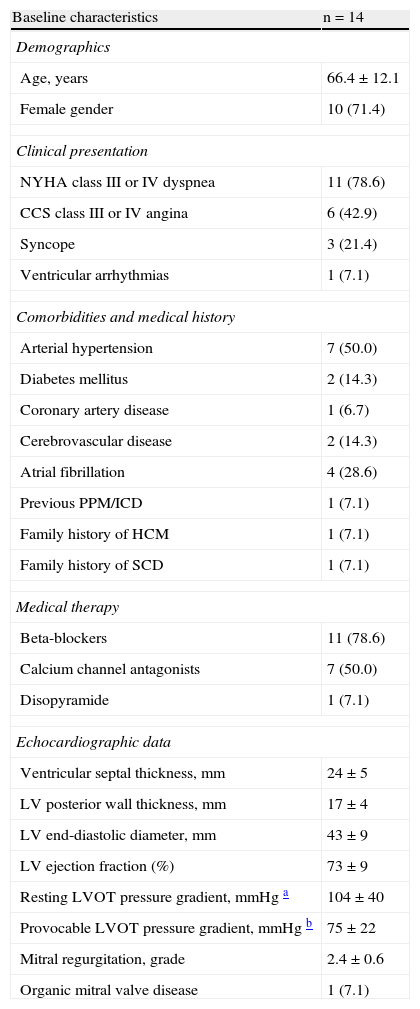
ResultsBaseline characteristicsPercutaneous ASA procedures were performed in 14 of 17 pre-selected patients with HOCM. In two patients the procedure was aborted due to echocardiographic evidence of echo-contrast staining of a large myocardial area, involving the basal and mid ventricular septum, with the consequent risk of serious complications; in a third patient, the procedure was interrupted before septal ablation due to a serious complication (see Procedural outcome). The baseline demographic, clinical and echocardiographic characteristics of the 14 patients who performed ASA (age 66.4±12.1 years; range 48–83 years; 71.4% women) are depicted in Table 1. All patients had severe cardiovascular symptoms (NYHA class III/IV dyspnea, 78.6%; CCS class III/IV angina, 42.9%). Significant LVOT obstruction on echocardiography was present at rest in the majority of patients (n=11, mean peak LVOT gradient 104±40mmHg), being provocable in the others (n=3, mean peak LVOT gradient 75±22mmHg on Valsalva strain).
Baseline characteristics of the population undergoing percutaneous alcohol septal ablation.
| Baseline characteristics | n=14 |
| Demographics | |
| Age, years | 66.4±12.1 |
| Female gender | 10 (71.4) |
| Clinical presentation | |
| NYHA class III or IV dyspnea | 11 (78.6) |
| CCS class III or IV angina | 6 (42.9) |
| Syncope | 3 (21.4) |
| Ventricular arrhythmias | 1 (7.1) |
| Comorbidities and medical history | |
| Arterial hypertension | 7 (50.0) |
| Diabetes mellitus | 2 (14.3) |
| Coronary artery disease | 1 (6.7) |
| Cerebrovascular disease | 2 (14.3) |
| Atrial fibrillation | 4 (28.6) |
| Previous PPM/ICD | 1 (7.1) |
| Family history of HCM | 1 (7.1) |
| Family history of SCD | 1 (7.1) |
| Medical therapy | |
| Beta-blockers | 11 (78.6) |
| Calcium channel antagonists | 7 (50.0) |
| Disopyramide | 1 (7.1) |
| Echocardiographic data | |
| Ventricular septal thickness, mm | 24±5 |
| LV posterior wall thickness, mm | 17±4 |
| LV end-diastolic diameter, mm | 43±9 |
| LV ejection fraction (%) | 73±9 |
| Resting LVOT pressure gradient, mmHg a | 104±40 |
| Provocable LVOT pressure gradient, mmHg b | 75±22 |
| Mitral regurgitation, grade | 2.4±0.6 |
| Organic mitral valve disease | 1 (7.1) |
Values are presented as number (%) or as mean±standard deviation, unless otherwise stated. CCS: Canadian Cardiovascular Society; HCM: hypertrophic cardiomyopathy; ICD: implantable cardioverter-defibrillator; LV: left ventricular; LVOT: left ventricular outflow tract; NYHA: New York Heart Association; PPM: permanent pacemaker; SCD: sudden cardiac death.
Percutaneous ASA procedures were performed in 14 patients through the injection of 3.1±1.1ml of ethanol in a mean of 1.1±0.5 perforator septal branches. Invasive LV outflow tract peak pressure gradient decreased from 87±34mmHg at baseline (provoked peak gradient of 153±32mmHg) to 30±23mmHg immediately post-ASA (p=0.001). The acute success rate of ASA was 71.4%. Procedural complications occurred in 6.7% of patients – one case of left anterior descending coronary artery dissection while attempting to engage the septal branch with the guidewire, the procedure being interrupted (ASA not performed). Pacemaker implantation due to iatrogenic third-degree atrioventricular block was performed in four patients (28.6%). A significant rise in troponin T level was observed in all patients (mean peak level 62±60ng/ml).
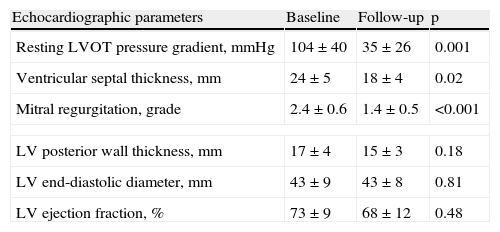
Echocardiographic evolutionThe long-term success rate among patients undergoing ASA was 85.7%. Echocardiographic resting peak LVOT gradient decreased from 104±40mmHg at baseline to 58±30mmHg post-procedure (p=0.03) and to 35±26mmHg at follow-up (p=0.001); total gradient decrease was 75±43mmHg (Figure 3). Ventricular septal thickness also decreased significantly during follow-up, from 24±5mm to 18±4mm (p=0.02). Mitral regurgitation showed a significant improvement, from grade 2.4±0.6 to 1.4±0.5 (p<0.001); only four patients remained with grade 2 mitral regurgitation. No significant alterations were found in any of the other echocardiographic parameters analyzed (Table 2).
Evolution of echocardiographic parameters during follow-up.
| Echocardiographic parameters | Baseline | Follow-up | p |
| Resting LVOT pressure gradient, mmHg | 104±40 | 35±26 | 0.001 |
| Ventricular septal thickness, mm | 24±5 | 18±4 | 0.02 |
| Mitral regurgitation, grade | 2.4±0.6 | 1.4±0.5 | <0.001 |
| LV posterior wall thickness, mm | 17±4 | 15±3 | 0.18 |
| LV end-diastolic diameter, mm | 43±9 | 43±8 | 0.81 |
| LV ejection fraction, % | 73±9 | 68±12 | 0.48 |
Values are presented as mean±standard deviation. LV: left ventricular; LVOT: left ventricular outflow tract.
Two deaths were recorded in the study population, both in-hospital (14.3%). One patient had a high-risk profile at the time of ASA (male, 83 years old, comorbidities, presentation with NYHA class III dyspnea and syncope, evolution in cardiogenic shock refractory to medical therapy, and rejected for surgical myectomy due to excessive risk); in addition, the procedure was unsuccessful (residual LVOT gradient 104mmHg). The patient evolved without clinical improvement, and died on day 9 post-procedure after an episode of ventricular fibrillation. The second patient was a 66-year-old woman with HOCM, admitted to the Emergency Department due to an episode of cardiorespiratory arrest in ventricular fibrillation, successfully resuscitated, in the context of NYHA class III dyspnea. Although percutaneous ASA was successful (acute fall of peak LVOT gradient from 83 to 37mmHg) and there were no procedural complications, the patient's clinical status did not improve, with evolution to cardiogenic shock and death in ventricular fibrillation 24 hours after the procedure.
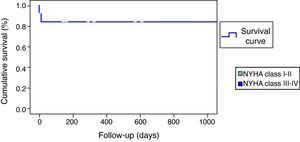
The remaining 12 patients had a largely uneventful short and long-term follow-up; only one patient suffered a transient ischemic attack in-hospital, without neurologic sequelae. No other deaths or ventricular arrhythmias were recorded during the mean 25-month clinical follow-up (Figure 4).
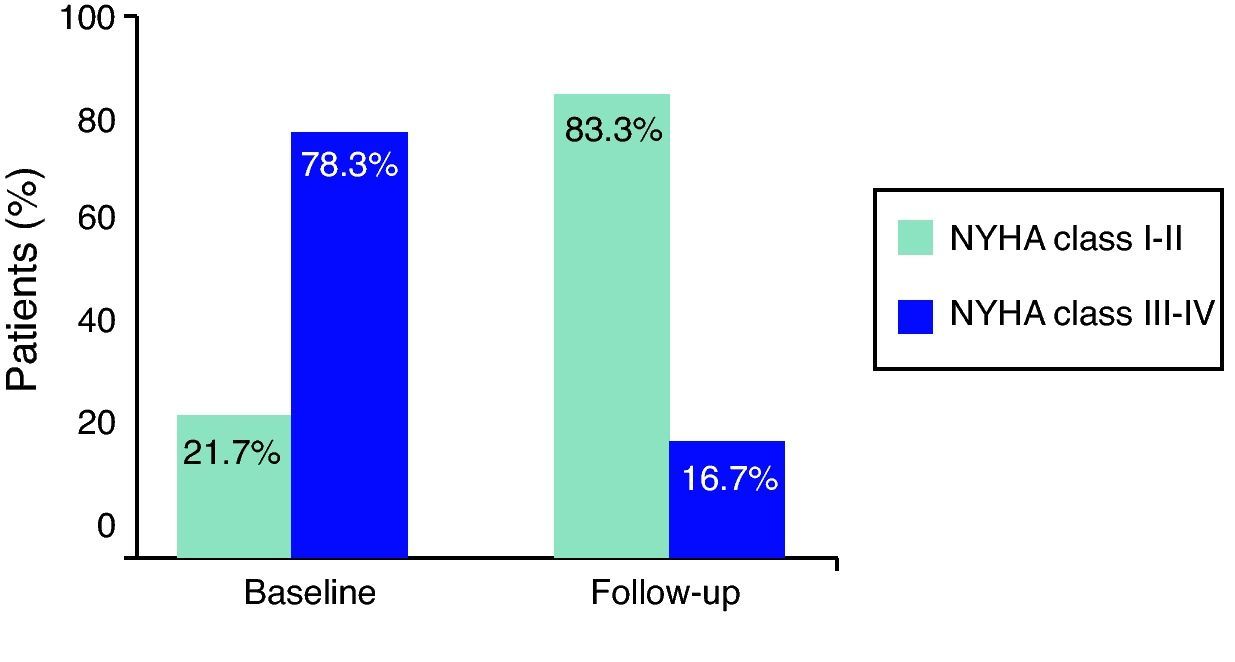
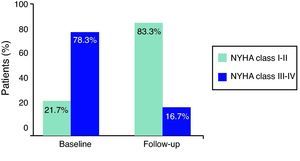
Marked symptom improvement was also seen: dyspnea evolved from a mean NYHA functional class of 2.6±1.1 to 1.8±1.4 during follow-up (p=0.09); functional class improved in 81.8% of patients, from 78.3% of patients in class III–IV at baseline to 83.3% in class I–II at the end of follow-up (Figure 5). No recurrence of angina or syncope was observed.
DiscussionOur results indicate that percutaneous alcohol septal ablation is an effective technique in the treatment of HOCM, in most patients (71.4%) yielding a significant acute fall in LVOT pressure gradient. This effect continues to progress throughout follow-up in most patients (85.7%), due to the gradual reduction in ventricular septal wall thickness secondary to alcohol-induced septal necrosis and consequent ventricular remodeling, with consequent clinical improvement in patients’ functional class.
Echocardiographic analysisOur short- and long-term echocardiographic outcomes of the efficacy of ASA – significant reductions in LVOT pressure gradient, ventricular septal thickness and mitral regurgitation, without a significant drop in LV ejection fraction – were very positive.
This evolution is in general agreement with the published data. A systematic review by Alam et al. in 2006 analyzed the results of ASA in 42 studies involving 2959 patients.9 Echocardiographic analysis showed a sustained decrease at 12 months in resting LVOT gradient (65.3 to 15.8mmHg, p=0.001) with an associated reduction in basal septal diameter (20.9 to 13.9mm, p=0.001); LV ejection fraction decreased from 74.5% to 67.4% (p=0.001), with slight increases in LV end-diastolic (from 44.5mm to 45.8mm, p=0.001) and end-systolic (from 23.3mm to 26mm, p=0.001) diameters. It is generally agreed by most authors that 70–90% of treated patients show a significant acute LVOT gradient reduction; most of this acute fall likely represents stunning and consequent hypo- or akinesia of the basal septum, and may be followed by a recurrence of the gradient 1–3 days after ASA. Then, a permanent and more substantial reduction in the gradient develops progressively within 3–12 months of the procedure, the direct result of scar formation and thinning of the basal septum and of a LV remodeling process that appears to extend to the entire myocardium, leading to regression of LV hypertrophy. Indeed, total LV mass decreases after septal ablation and this reduction exceeds that of septal mass, findings that can be regarded as a result of the elimination (or at least reduction) of the pressure overload.10 A significant and sustained improvement in echocardiographic diastolic parameters post-ASA has also been shown.11 Furthermore, and as seen in our study, ASA has been associated with a significant decrease and even abolition of systolic anterior motion and mitral regurgitation in long-term follow-up, and with a decrease in systolic pulmonary artery pressure.12 It is therefore prudent to allow for that time course before the treatment is deemed ineffective and any decision about repeat intervention is made.
Clinical outcome and complicationsThe most impressive clinical result of our study was the clear symptomatic improvement of patients undergoing ASA: NYHA functional class improved in 81.8% after the procedure, only two patients remaining in NYHA class III (14.3%), and no cases of angina or syncope recurrence were observed during the mean 25-month clinical follow-up. Cardiac structural and functional changes are associated with a significant symptomatic improvement in HOCM patients undergoing percutaneous ASA. NYHA class is significantly lower after three months, with ongoing improvement during the first year.12,13 The above-mentioned systematic review showed a significant decrease in NYHA functional class (2.9 to 1.2, p=0.001) and angina (mean CCS class from 1.9 to 0.4, p=0.001), as well as an increase of 34% in mean treadmill exercise capacity (p=0.001) and of 33% in peak oxygen consumption (p=0.001) at 1-year follow-up.9
However, ASA is an interventional procedure and involves a non-negligible risk of complications. Serious complications were relatively uncommon in the same systematic review: early (30-day) mortality was 1.5% with a late mortality of 0.5%; ventricular fibrillation in the immediate preoperative period occurred in 2.2%, LAD dissection in 1.8%, and pericardial effusion in 0.6%. The most frequent complications were conduction disturbances: new right bundle branch block developed in 46% of patients and first degree atrioventricular block in 53% following ASA; permanent complete heart block requiring permanent pacemaker occurred in 10.5% of the patients treated. Rarer problems were coronary artery spasm, cardiogenic shock, pulmonary embolism and stroke.9
Our in-hospital rate of complications was, in general, higher than that described: one procedural LAD dissection (6.7%) and three major in-hospital clinical events (two deaths and one transient ischemic attack, 21.4%). By contrast, no serious events (including death) occurred after the first 30 days. Our rate of permanent pacemaker implantation due to persistent third-degree atrioventricular block was also high (28.6%). While these numbers are cause for concern, three main factors may have influenced them: patient selection, the learning curve of the technique, and the small population size. Both deceased patients were very high-risk cases: the first presented in refractory cardiogenic shock, ASA having been performed with strictly life-saving objectives, while the second had been admitted due to cardiorespiratory arrest in ventricular fibrillation. While they may reflect real-world practice, these patients are not representative of those included in clinical trials. The learning curve is also an unavoidable issue when a first series of cases of any technique is presented; percutaneous ASA is a complex procedure, in which most of the complications occur in the catheterization laboratory or during the early post-interventional period, and a moderate to extended learning curve effect is to be expected. While earlier series have shown in-hospital mortality rates of around 4%, these have now fallen to around 1.5%. It seems clear that improved expertise might be an integral part of refinement of the procedure, with subsequent falls in the complication rate.9,14 Technical advances, reflected in our series (which represents 10 years of evolution), have also influenced ASA prognosis: for instance, the introduction of echocardiographic contrast guidance has essentially eliminated the risk of inducing apical or lateral wall infarcts due to the presence of septal collaterals to these areas, and the use of lower doses (≤2ml) of ethanol has been associated with lower incidence of permanent pacemaker implantation and greater survival benefit.15,16 Finally, it should not be overlooked that the small size of the sample analyzed makes it difficult to discount the effect of chance on the results.
Percutaneous alcohol septal ablation versus surgical myectomyWhile this was not the aim of our study, it important to contextualize our results with those obtained by alternative techniques. Since its introduction by Sigwart in 1994, ASA has been performed in thousands of patients with HOCM with good short-term results.5 However, there have been no randomized control trials comparing the efficacy and outcomes of percutaneous ASA and surgical myectomy. Several non-randomized comparative studies were recently analyzed in both a meta-analysis (Alam et al., 2009, involving five studies and 351 patients) and a systematic review (Leonardi et al., 2010, involving 27 studies and 4094 patients).7,8 The results show that both therapeutic modalities offer similar significant reductions in LVOT obstruction and symptomatic improvement, as well as similarly low all-cause in-hospital and long-term mortality and sudden cardiac death. Myectomy is associated with a lower risk of complete AV block necessitating permanent pacemaker implantation, although at the cost of a higher risk of stroke and a longer postoperative recovery period; following ASA, a higher number of patients required a second procedure (either repeat ASA or myectomy) because of an unsatisfactory result.17,18 Therefore, in view of these emerging long-term results, surgery and percutaneous septal ablation should be regarded as alternative treatment options in HOCM, in terms of safety and efficacy. It is prudent to take account of the benefits and drawbacks of each therapeutic method when deciding on treatment for LVOT obstruction, taking into consideration clinical, morphological, and technical aspects, the individual experience of the center and the merit of each treatment modality for the individual patient.
Study limitationsThe present study has a retrospective design, and intervened patients were subject to selection bias, which must be considered in the interpretation of its results. Most importantly, the small population analyzed limited the statistical analysis of the results and their extrapolability. Despite these limitations, our study benefits from technical consistency between patients and a careful long-term clinical and echocardiographic follow-up, factors reflected in significant results that are very similar to the published data. Most of all, it was never our intention to analyze a representative population or to reach innovative conclusions, but only to perform and share a critical self-evaluation of our initial practice in this area.
ConclusionsPercutaneous ASA was shown to be an effective alternative therapy in patients with severe symptomatic HOCM refractory to medical treatment. In this population it was associated with significant acute and long-term falls in LVOT pressure gradient, ventricular septal thickness and mitral regurgitation, accompanied by clear functional improvement in long-term follow-up. However, this invasive technique was not free of procedural and periprocedural complications, underlining the importance of careful patient selection and of the technical expertise of the center. Alcohol septal ablation is still evolving, and it is hoped that refinements in the technique will lead to further improvement in outcomes.
Conflicts of interestThe authors have no conflicts of interest to declare.
Dr. Sílvio Leal wishes to express his gratitude to the Portuguese Society of Cardiology, from whom he was receiving a training grant at the time this article was written.