Degenerative mitral valve disease (myxomatous degeneration or fibroelastic deficiency) is the most common indication for surgical referral to treat mitral regurgitation. Mitral valve repair is the procedure of choice whenever feasible and when the results are expected to be durable. Posterior leaflet prolapse is the commonest lesion, found in up to two-thirds of patients. It is the easiest to repair, particularly when limited to one segment. In these cases, rates of repairability and procedural success approach 100%, and there is now ample evidence that the immediate and long-term results are better than those of valve replacement. Notably, minimally invasive valvular procedures, surgical or interventional, have attracted increasing interest in the last decade. When performed by experienced groups, mitral valve repair is unrivaled irrespective of the severity of lesions, from simple to complex, which leaflets are involved, and the type of degenerative involvement (myxomatous or fibroelastic). Its results should be viewed as the benchmark for other present and future technologies. By contrast, percutaneous mitral valve repair is still in its infancy and its results so far fall short of those of surgical repair. Nevertheless, continued investment in transcatheter procedures is of great importance to enable development and improved accessibility, particularly for patients who are considered unsuitable for surgery. In this review, we analyze the current status of management of degenerative mitral valve disease, discussing mitral valve anatomy and pathology, indications for intervention, and current surgical and transcatheter mitral valve procedures and results.
A doença valvar mitral degenerativa – deficiência mixomatosa ou fibroelástica – é a indicação mais comum de referência cirúrgica para tratamento da regurgitação mitral. A plastia da válvula mitral é o procedimento de escolha sempre que possível e os resultados são duradouros. O prolapso do folheto posterior é a lesão mais comum, encontrada em cerca de dois terços dos pacientes. É mais fácil de reparar, principalmente quando limitado a um segmento. Nesses casos, as taxas de reparabilidade e de sucesso aproximam-se dos 100% e agora há ampla evidência de que os resultados imediatos e a longo prazo são melhores do que os da substituição valvular. Os procedimentos valvares minimamente invasivos, cirúrgicos ou de intervenção, têm ganhado crescente interesse na última década. Quando realizada por grupos experientes, a plastia da valva mitral não tem rival, independentemente da gravidade das lesões, das simples às complexas, de quais os folhetos envolvidos e do tipo de patologia degenerativa (deficiência mixomatosa versus fibroelástica). Os resultados devem ser usados como referência para outras tecnologias presentes e futuras. Por outro lado, o tratamento percutâneo da válvula mitral ainda é incipiente e os resultados, até ao momento, ficam aquém dos da correção cirúrgica. No entanto, o investimento contínuo em procedimentos transcateter é de extrema importância para possibilitar a evolução e acessibilidade aos pacientes, principalmente àqueles aos quais é recusada a cirurgia. Nesta revisão, analisamos o estado atual do tratamento da doença degenerativa da válvula mitral, discutindo a anatomia e patologia valvular, as indicações para a intervenção, os procedimentos e resultados cirúrgicos e transcateter atuais.
Mitral regurgitation (MR) is one of the most frequent valvular lesions, with a prevalence of more than 10% in individuals older than 75 years.1,2 Degenerative mitral valve (MV) disease (DMVD) (myxomatous degeneration or fibroelastic deficiency) is the most common indication for surgical referral to treat MR.
Mitral valve repair (MVr) is the procedure of choice whenever feasible and when the results are expected to be durable.3 MVr can be said to require a state of mind, in which the surgeon must truly believe in the superiority of this treatment to overcome all barriers to achieve the perfect repair without compromising the patient. There is substantial evidence providing a solid basis for the surgeon to opt for repair, rather than replacement, and not base his or her preference on mere intuition.4–6 Unfortunately, no randomized controlled trials have been conducted comparing the two treatments, probably due to the lack of equipoise between them.
Notably, minimally invasive valvular procedures, surgical or interventional, have attracted increasing interest in the last decade. Transcatheter aortic valve implantation (TAVI) has paved the way for modern structural heart valve interventions in this era, as balloon mitral valvuloplasty had done previously, almost 40 years ago.7 After the exponential growth of TAVI, as well as of minimally invasive surgical procedures (partial sternotomy or right anterior minithoracotomy), to address severe aortic valve disease,8,9 there has been renewed interest in the MV over the past few years, particularly in mitral regurgitation. The development of new technologies has enabled intervention in patients previously deemed too ill for treatment, and this has also brought the MV to the forefront of heart valve interventions.
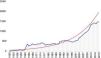
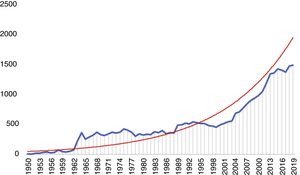
There has accordingly been a significant increase in the number of publications on the MV, the majority of which focus on mitral regurgitation and MV surgery or percutaneous intervention (Figure 1). In this review, we analyze the current status of management of DMVD, discussing MV anatomy and pathology, indications for intervention, and current surgical and transcatheter MV procedures and results.
Anatomy of the mitral valve: a common language for surgeons and cardiologistsThe MV is a complex anatomical structure whose two leaflets separate the left atrial and ventricular cavities. It would in fact be more accurate to define it as the mitral valvar complex, since its composition goes beyond the two leaflets, also comprising the annulus, the chordae tendineae and the papillary muscles.10 Importantly, the myocardium from which the papillary muscles originate participates in a crucial interplay with the valve apparatus, and any imbalance may eventually lead to mitral regurgitation.
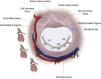
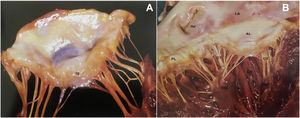
LeafletsThe two leaflets, anterior (septal or aortic) and posterior (mural), are markedly different in shape and size and are inserted as a continuous veil around the entire circumference of the mitral orifice.11 The anterior leaflet is larger and triangular, with a thin translucent appearance at the base and body (the clear zone), but has a thicker opaque crescent-shaped area bordering the free edge (the rough zone), which is the main area of chordal attachment and of coaptation (Figure 2A). It is in fibrous continuity with the left and non-coronary cusps of the aortic valve and with the interleaflet triangle between the aortic cusps that borders the membranous septum.12
(A) The greater thickness of the rough zone (rz) of the anterior mitral valve leaflet; (B) posterior view of the open mitral valve, between the left atrium (LA) and the left ventricle (LV) (from 13). Ap: left atrial appendage; AL: anterior leaflet; CT: chordae tendineae; PL: posterior leaflet; PM: papillary muscle.
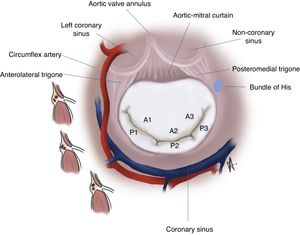
The posterior leaflet is narrower but has a longer attachment to the annulus, covering two-thirds of the entire perimeter (Figure 2B). It has indentations (also called clefts) that generally delimit three scallops or segments along the elongated free edge. Carpentier denoted the most lateral segment (which lies adjacent to the anterolateral commissure) as P1, the central segment as P2, and the segment adjacent to the posteromedial commissure as P3.14 The anterior leaflet is also divided arbitrarily into three segments labeled A1, A2 and A3, corresponding to the adjacent regions of the posterior leaflet (Figure 3).
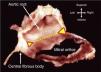
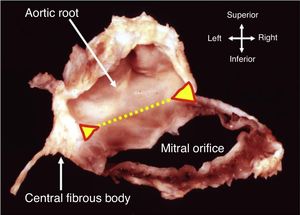
AnnulusThe annulus is an ill-defined fibromuscular ring, D-shaped rather than circular, that serves to anchor the leaflets. The right and left fibrous trigones, both structures of the cardiac skeleton, are part of the anterior aspect of the MV ring and are interconnected by the aortic-mitral curtain (Figure 4). This region of the annulus is thus fibrous and less prone to dilate. Beyond this point, in the remaining two-thirds, the annulus is mainly muscular. In significant mitral regurgitation, this is the portion most prone to dilatation, as well as to calcification.
Dissection showing how part of the annulus is formed by the region of fibrous continuity with the leaflets of the aortic valve (dotted yellow line). The ends of this area of continuity (triangles) are the fibrous trigones that anchor the valve complex to the roof of the left ventricle. The right trigone merges with the membranous septum to form the central fibrous body (from 10).
Interestingly, the posterior annulus is described in different ways depending on whether the observer is an anatomist, a surgeon, or an imager.15 The first describes it as the convergence of four components: the atrial wall, the leaflet hinge line, the crest of the left ventricular (LV) free wall and the epicardial adipose tissue of the atrioventricular sulcus. The surgeon distinguishes the atrial wall, for its slightly pink color, from the posterior leaflet, for its yellowish color. Thus, the posterior annulus is a virtual line that separates the atrial wall from the posterior leaflet. Finally, the different imaging modalities have distinct abilities to image the posterior annulus, emphasizing different aspects of its anatomical structure. Two-dimensional (2D) transthoracic (TTE) and transesophageal (TEE) echocardiography, computed tomography (CT) and cardiac magnetic resonance imaging share the same cross-sectional views, and the string of fibrous tissue appears as a fibrous nodule. However, neither 2D TTE nor TEE can clearly distinguish the fibrous nodule from epicardial adipose tissue or from muscular tissue, hence from several aspects CT is more informative.15
The annulus is a three-dimensional (3D) structure, not flat, and its shape and size vary with the different phases of the cardiac cycle. Its morphophysiology can be profoundly altered both primarily and secondarily to disease of the valve-left ventricle complex. Severe myxomatous involvement is frequently associated with excessive mobility of the leaflets, which in turn is usually secondary to disjunction of the cardiac skeleton. Mitral annular disjunction is characterized by separation between the atrial wall-MV junction and the LV attachment.16
Chordae tendineae and papillary musclesAs the name implies, the chordae tendineae are tendinous structures which originate from the tip of the papillary muscles and insert into the ventricular side of the valve leaflets, although some originate directly from the ventricular wall. The chordae tendineae are usually fan-shaped, branching before leaflet insertion, but some may insert directly into the leaflet. Traditionally, they are classified as primary chords, when attached to the free edge of the leaflets; secondary, attached to the ventricular surface in the region of the rough zone (i.e. the body of the leaflet); and tertiary, inserted at the base of the posterior leaflet only.
The two papillary muscles are extensions of the ventricular myocardium and contract and relax with it. They are generally described as anterolateral and posteromedial and originate from the mid to apical segments of the left ventricle. Although the anterior papillary muscle may remain single, it usually bifurcates, while the posterior muscle can have up to three heads in the majority of adults. However, this distribution can vary significantly, particularly in patients with myxomatous leaflets.17
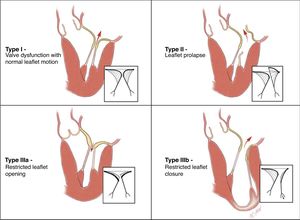
Pathology of mitral valve regurgitationThe lack of a universal nomenclature to define DMVD is a significant barrier to the interpretation of studies aimed at assessing MV interventions. Carpentier proposed a pathophysiological triad that gave an important insight into the genesis of MR, enabling a methodological approach to treatment.18 The etiology (cause of the disease) leads to the appearance of lesions (consequence), which in turn causes dysfunction (effect). Carpentier's classification of valve dysfunction based on leaflet motion – type I: normal leaflet motion (annular dilatation); type II: excessive motion (prolapse); and type III: restricted motion (Figure 5) – is now widely accepted. This analysis also has important prognostic implications, since the results of MVr (repairability and durability) vary between different etiologies, type of valve dysfunction and lesions encountered (site of prolapse, presence of calcification, leaflet restriction, etc.).
Carpentier's functional classification (from 18).
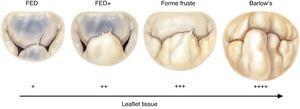
The most frequent types of DMVD encountered in developed countries are Barlow's disease and fibroelastic deficiency. These are at the opposite ends of the spectrum of degenerative pathology of the MV (Figure 6), differing not only in morphologic features but also in clinical presentation. For instance, patients with Barlow's disease tend to be younger, with a known history of MR (usually more than 10 years) and with characteristic features such as tissue excess and thickening (cauliflower appearance), multiple prolapsing segments (usually with chordal elongation) and involving both leaflets, severely enlarged annulus, and calcification.
Spectrum of degenerative mitral valve disease (from 19). FED: fibroelastic deficiency.
By contrast, patients with fibroelastic deficiency are older (>60 years), with a recent history of symptoms and with a normal quantity of leaflet tissue in a normal-sized annulus. The leaflets are typically thin and the chordae are flimsy. Regurgitation is typically caused by elongation and/or rupture of one or more chordae in a single prolapsing segment, most often P2. In the chronic setting, the prolapsing segment may become distended and thickened by a limited myxomatous process.20
Indications for mitral valve surgeryMitral valve surgery is the only recommended treatment (class I or IIa) for DMVD.3,21 Interestingly, the level of evidence is B or C, which means that no randomized trials have been performed that clearly show the superiority of MVr or of early mitral valve surgery.
The timing for surgery in severe primary MR (MVr is preferred) is clearly stated in medical society guidelines and consensus documents. Patients should be operated (class I indication) when symptomatic and with left ventricular ejection fraction (LVEF) >30%, or when asymptomatic and with signs of LV dysfunction (LV end-systolic diameter [LVESD] >40 mm [American guidelines] or >45 mm [European] and/or LVEF <60%). Surgery should be considered (class IIa recommendation) in asymptomatic patients with preserved LV function (LVESD <45 mm and LVEF >60%), when there is atrial fibrillation (AF) secondary to MR or pulmonary hypertension (systolic pulmonary pressure >50 mmHg at rest).3 Additionally, it should also be considered when a durable repair is likely, surgical risk is low, the repair is performed in a heart valve center and at least one of the following findings is present: flail leaflet or presence of significant left atrial dilatation (volume index ≥60 ml/m2 body surface area) in sinus rhythm.3
The natural history of severe chronic MR shows that untreated patients will inevitably develop symptoms of heart failure, LV dysfunction, AF or pulmonary hypertension (Figure 7A), and when operated at that time they do worse than those without these features (Figure 7B). Furthermore, there is overwhelming consistent and cumulative evidence worldwide that early surgery should be the preferred management for organic MR.22–27
Natural history of mitral regurgitation (MR) and optimal timing for surgery. (A) Severe MR will inexorably lead to left ventricular (LV) dysfunction and/or dilatation, symptoms, atrial fibrillation (AF), pulmonary hypertension (PHT), and eventually death. The optimal timing for surgery would be before the decompensated stage when irreversible damage can supervene; (B) survival curves comparing asymptomatic or mildly symptomatic patients with patients in New York Heart Association (NYHA) III-IV at the time of surgery, in our experience (from 28).
There are various factors that make MVr the gold standard treatment for severe MR. Firstly, MVr confers superior outcomes to valve replacement and improves life expectancy.6,29–35 These demonstrated advantages of MVr include better short- and long-term survival,6 improved quality of life, better preservation of left ventricular function, and greater freedom from endocarditis and anticoagulant agent-related bleeding.5 MVr is possible in >90% of patients with degenerative mitral valve disease36–43 and can be achieved with an operative mortality risk of <1%, in some centers even approaching 0%.44–47
Secondly, there are several other reasons for early surgery48:
- 1.
In patients with organic MR, surgery is almost unavoidable. Patients with severe organic MR have measured rates of death or need for cardiac surgery ranging from 10%-30% per year, meaning that 10 years after diagnosis, 90% of these patients will either be dead or have undergone surgery.49–51
- 2.
Class I indications for mitral surgery are associated with dire outcome consequences. Patients operated when in a class I indication for surgery exhibit markedly higher operative and late mortality. For instance, having symptoms at the time of surgery is associated with an 80% increase in late mortality compared with those with no or minimal symptoms.27,49,52
- 3.
Organic MR is a condition with serious consequences. All studies of the natural history of MR show that patients with moderate to severe MR have 3% annual mortality, compared with 6% for severe MR. In addition, several studies have demonstrated high rates of cardiac events (cardiac death, heart failure, AF), which approach 10% per year in severe MR.27,53–55
- 4.
No alternative treatment of organic MR is better than surgery. There is no doubt that medical therapy cannot correct severe MR or postpone surgery indefinitely. Notably, despite the promising results of percutaneous therapy, such as with the MitraClip, it is still limited in the range of technical options to correct the variety of lesions presented in degenerative MR. As discussed below, the data regarding the long-term durability of percutaneous repair are scarce, but it is widely recognized that the Alfieri stitch or edge-to-edge technique (the surgical precursor of the MitraClip) had poor outcomes if not accompanied by reliable annuloplasty, which is still not achievable by percutaneous intervention (see below).56,57
- 5.
Comparative studies favor early surgery. The strategy of watchful waiting, proposed by Rosenhek and colleagues,58 is based on the assumption that asymptomatic patients with severe degenerative MR can be regularly followed with clinical and echocardiographic examinations and sent to surgery as soon as they reach the indications recommended by the guidelines, without compromising survival. Six-month follow-up intervals are recommended and this timeframe can be further shortened if deemed necessary. However, the initial signs of incipient decompensation may be overlooked by insufficiently rigorous outpatient review and the onset of symptoms can be rather insidious and remain undetected, particularly in more sedentary patients. The majority of groups and multicenter studies, such as the Mitral Regurgitation International Database, have clearly demonstrated improved outcomes, from survival to other morbid conditions (AF, pulmonary hypertension, LV dysfunction, etc), with an early strategy compared to a wait-and-see approach.22,27,41,59–61
The state of the art dictates that the operation should be tailored to the patient and not the patient to the operation.
For more than three decades, the philosophy has been that all degenerative mitral valves are, in principle, amenable to repair, provided that a thorough and comprehensive pre- and intraoperative analysis of the whole valve apparatus is performed.28 Accurate identification of all lesions responsible for the valve dysfunction is of paramount importance because it enables selection of appropriate surgical techniques. Modern reconstructive MV surgery is based on three main goals, in order to provide not only a functional but also an anatomical approach: to restore or preserve appropriate leaflet mobility; to ensure good leaflet coaptation; and to remodel and stabilize the annulus.
The techniques used have evolved over time. Some have completely disappeared, others have been refined and newer ones have been added to the surgeons’ armamentarium. It is beyond the scope of this work to describe them in detail and they are now well known even by non-surgeons. Table 1, based on our own experience,21 shows the lesions most frequently encountered in degenerative MR and the surgical techniques used to correct them, in addition to the probability of repair.
Lesions found in degenerative mitral valve disease and the surgical techniques used to correct them.
| Lesion | Surgical techniques | Probability of repair |
|---|---|---|
| Annular dilatation | Annuloplasty procedure: | >95% |
| complete ringa | ||
| partial ring/bandb | ||
| suture annuloplastyc | ||
| Posterior leaflet prolapse | Artificial chordal implantationa | >98% |
| Leaflet resectiona | ||
| Sliding plastyb | ||
| Notch closure between segmentsb | ||
| Chordal shortening/transpositionc | ||
| Anterior leaflet prolapse | Artificial chordal implantationa | >95% |
| Chordal shortening/transpositionb | ||
| Suture plication (minor prolapse)b | ||
| Leaflet resectionc | ||
| Commissural leaflet prolapse | Commissural closure (‘magic stitch’)a | >95% |
| Papillary muscle shorteningb | ||
| Artificial chordal implantationb | ||
| Chordal shortening/transpositionc | ||
| Leaflet restriction/small size | Patch augmentationb | 70%-80% |
| Leaflet thinningb | ||
| Secondary chordal resectionb | ||
| Annular calcification | Decalcificationb | 70%-80% |
| Decalcification+patch reconstructionc |
The following options express the authors’ opinions and tendencies according to their daily experience.
Pre-, intra- and postoperative imaging, especially echocardiography, is of the utmost importance in predicting mitral valve repairability, evaluating the surgical result and predicting the durability of the repair. TTE, or TEE when the patient has a poor acoustic window or TTE cannot accurately determine MR severity or clearly reveal the mechanism of regurgitation, are the cornerstones of diagnostic assessment. There are several echocardiographic features that should be studied in order to prepare for surgery: annulus size and mobility (mitral annular disjunction, calcification); leaflet characteristics (dimensions – height and thickness, diseased segments, calcification); and chordae (elongated, ruptured), as well as the rest of a standard echocardiographic examination. Intraoperative TEE is mandatory in mitral valve surgery and 3D imaging can play an important role in identifying diseased segments, location of MR jets and calcification. The definition of a good repair is complete elimination of or, at most, residual MR. Other important objectives are a good leaflet coaptation surface (length >10 mm), restoration of normal leaflet mobility, good MV opening (avoiding a restrictive valve with a significant diastolic gradient), and absence of systolic anterior motion (SAM).
Posterior leaflet prolapse, especially of the P2 segment, is found in almost 75% of patients with severe degenerative MR. When isolated, this is probably the simplest to repair, especially in fibroelastic deficiency when there is a single ruptured chorda. We and others have demonstrated that a nearly 100% repair rate of posterior leaflet prolapse can be expected, with long-term durability.36,42 Although the current trend is to respect the native leaflet using artificial chordae instead of resecting a segment, the best option may be to “respect when you can and resect when you should.”62 Repair rates for anterior leaflet or bileaflet prolapse are usually slightly lower than for posterior leaflet prolapse, but experienced centers report rates above 90%-95%, also with low mortality (<1%).40,43,63
There are several key points that deserve particular attention when repairing Barlow valves. The height and volume of a large posterior leaflet should be reduced by resection (with or without sliding) or chordal implantation (lowering the free margin well into the ventricle) in order to avoid SAM; artificial chordae should be preferred, instead of the classical Carpentier shortening or transfer techniques to correct anterior leaflet prolapse; in bileaflet prolapse, start by correcting posterior leaflet prolapse; and favor the use of large rings (>34 mm).
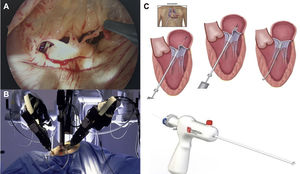
Minimally invasive mitral valve surgeryThe term minimally invasive mitral valve surgery (MIMVS) does not refer to a single procedure but rather to a series of methods aimed at decreasing surgical trauma, by minimizing the size of the incisions and avoiding full sternotomy. Access for MIMVS can be divided into three groups: partial sternotomy, right minithoracotomy, and more recently left anterior minithoracotomy for transapical neochordal placement. This includes open and video-assisted methods (Figure 8A), with or without robotic assistance.
Minimally invasive mitral valve procedures. (A) Endoscopic view from a right minithoracotomy, showing ruptured chordae of P2 segment; (B) Da Vinci robot; (C) surgical steps of chordal implantation with the Harpoon device (left anterior minithoracotomy in beating heart without extracorporeal circulation72).
A recent Italian multicenter study showed that MIMVS has increased dramatically in recent years in 10 cardiac surgical centers in Italy, from 27.5% in 2011 to 71.7% in 2017.64 Axtell et al. showed excellent results in a series of 101 patients undergoing MVr with right minithoracotomy, with no operative mortality and 100% survival at one year.65 Interestingly, during the timeframe of their study, 181 patients underwent sternotomy; the reasons mentioned for not performing MIMVS in this series were the presence of moderate or greater aortic valve insufficiency, aortoiliac disease, significant mitral annular calcification, severe pectus excavatum, predicted operative mortality exceeding 4%, and previous cardiac surgery. In essence, the authors chose MIMVS for healthy patients who were more likely to have a successful procedure.
There have been several studies, including metanalyses, showing similar results between conventional surgery and MIMVS,66-69 and minimally invasive techniques appear to achieve comparable results even in complex cases.63,64 Of note, it seems that MIMVS is not associated with higher costs.70 Hawkins et al. reached the conclusion that greater surgical and implant costs with MIMVS were offset by decreased transfusions and ancillary needs, leading to equivalent overall hospital costs.67 However, it is important to stress that MIMVS should only be performed by experienced surgeons in high case-volume institutions.
Robot-assisted MIMVS (Figure 8B) is the least invasive approach, totally endoscopic and without significant rib spreading, but is associated with high capital investment, resulting in significantly higher per-case operative costs. Nonetheless, it has attracted renewed interest in recent years, particularly in the US, where several groups have presented excellent results.71 The Cleveland Clinic group recently published their early results in 1000 patients undergoing robotically-assisted mitral valve surgery.72 Repair was accomplished in 99.2% of patients. Nonetheless, this series represented only 30% of the total number of patients undergoing MV surgery during the same period. There was only one hospital death (0.1%) and pre-discharge echocardiography showed that MR was mild or less in 97.9% of patients. The advantages reported for this procedure are the superb 3D visualization of the valvular and subvalvular apparatus and the precise movements given by the EndoWrist, which permits complex surgical maneuvers with a high degree of dexterity.73
Transapical off-pump neochordal placement has recently emerged as an alternative to transcatheter procedures or for patients at high risk for surgery with extracorporeal circulation and MV prolapse.74–77 It consists of implanting a Gore-Tex neochord (W.L. Gore & Associates, Newark, DE) under TEE guidance on the prolapsing segment and securing it at a defined length on the LV apex (Figure 8). Colli et al.75 recently reported one-year outcomes with the NeoChord DS1000 Artificial Chordae Delivery System (NeoChord, Inc., St. Louis Park, MN), with promising results. In 144 consecutive patients (2013-2017), the procedural success rate was 98.6%; two patients underwent conversion to open surgery due to immediate failure and two high-risk patients died during hospitalization (1.4%). The primary endpoint, defined as moderate or less MR and freedom from reoperation at one year, was achieved in 124 patients (89%). This procedure is still at the experimental stage and is only recommended for high-risk patients.
Long-term outcomes – survival and durability of mitral valve repairMVr results have been scrutinized for more than four decades. Carpentier's seminal work on the “French correction”78 was published almost 40 years ago and his group presented the first long-term (>20 years) results in non-rheumatic disease nearly 20 years ago (1970-1984; n=162; mean age 56±10 years).79 The outcomes were remarkable and should be viewed as a benchmark for any kind of procedure on the mitral valve: low 30-day mortality (1.9%), excellent late survival (73.4% at 10 years and 48% at 20 years), and freedom from reoperation (posterior leaflet prolapse: 98.5% at 10 years and 96.9% at 20 years; anterior leaflet prolapse: 86.2% and 86.2%, respectively; bileaflet prolapse: 88.1% and 82.6%, respectively). In our experience, freedom from reoperation for posterior leaflet prolapse at 15 years was 97%42 and 88% for bilateral and anterior leaflet prolapse at 20 years.43 However, David and colleagues have cautioned that although reoperation is uncommon after MVr, there is an increasing incidence of recurrent MR, tricuspid regurgitation, and new AF over time.80
In surgery, the reproducibility of techniques that translate into similar outcomes is crucial for the transfer of knowledge to other surgeons, especially younger ones. MVr has evolved from a procedure within the reach of only a few surgeons to being part of the armamentarium of the majority (especially for P2 prolapse). In this regard, several groups,80–83 including ours,41–43 have presented results that are comparable and even superior to those initially presented by Carpentier's group. Theoretically, every case of degenerative MR is amenable to repair, particularly in high-volume centers with surgeons who are highly experienced in MVr.37,38 Individual surgeon and group experience plays a critical role when managing the most difficult cases, such as Barlow's disease. However, it should be recognized that various features, including calcification, multiple prolapsing segments, leaflet retraction or tethering, and fibrotic leaflets, can decrease the repairability rate and the durability of repair.
Currently, it is indisputable that a good repair restores life expectancy.21,34,36 Furthermore, this benefit has been observed even in elderly patients in comparison with mitral valve replacement.84 In some cases, however, valve replacement is in fact necessary. It is always preferable to have a properly functioning prosthesis than a poorly repaired valve.
Percutaneous mitral valve interventions – competitor or bail-out procedure?The number of transcatheter valve interventions has increased exponentially in recent years, driven mainly by the appeal of treating and being treated without opening the chest and by avoidance of cardiopulmonary bypass complications. Nevertheless, patients with isolated severe degenerative MR are usually at low risk, particularly when operated early, with few comorbidities – those who would otherwise be healthy individuals. Hence, in this setting, should we accept anything but a perfect repair, whichever procedure is chosen? Are all patients well-informed when accepting a procedure other than surgical MVr, which is, to date, the only treatment proven to restore life expectancy and demonstrating long-term durability of repair in DMVD?
Certain factors make it clear that percutaneous mitral repair with the MitraClip device is a palliative procedure rather than a curative treatment in DMVD.
Firstly, it only achieves a functional (reduction of the degree of MR) rather than a complete functional and anatomical recovery. It does not treat the other components of DMVD, such as annular dilation and/or dysfunction, excessive tissue, chordal length or fragility, intersegmental leaks (posterior indentations between P1-P2 and P2-P3), and calcification.
Secondly, it transforms a single-orifice valve into a double-orifice valve, thus reducing mitral valve opening, and is frequently associated with varying degrees of mitral valve stenosis (anatomical and functional).
Thirdly, it is associated with high recurrence of MR or MV intervention/reoperation in the medium term. In the EVEREST II trial, in which 73% of patients in the MitraClip arm had DMVD, freedom from reintervention at five years was only 43%, with an increased risk within six months of the procedure.85
Finally, as mentioned before, the edge-to-edge surgical technique, which the MitraClip and other techniques are intended to replace, is associated with more frequent MR recurrence whenever mitral annuloplasty is not performed. Surgical mitral annuloplasty (with complete or partial rings or bands) is invariably performed in all patients with degenerative MR. Percutaneous annuloplasty techniques and devices, either direct (Cardioband, Edwards Lifesciences) or indirect (Carillon, Cardiac Dimensions; Arto System, MvrX Inc.; and others) are in the initial stages of development and conflicting results have been reported.86,87
However, it is also indisputable that some patients with severe MR are considered unsuitable for surgery because of increased perioperative risk owing to advanced age, frailty, LV dysfunction, and comorbidities.88 This has triggered a dramatic growth in the development of novel transcatheter mitral valve technologies for repair and replacement.89 In this context, there has been increased interest in transcatheter mitral valve replacement (TMVR), because it reduces the risk of recurrent MR inherent to repair techniques. TMVR has wide application in high-risk situations, including dysfunction of mitral prosthesis (valve-in-valve), MVr failure (valve-in-ring), or mitral annular calcification.
ConclusionsDMVD is the most common etiology of severe MR in the Western world, and posterior leaflet prolapse is the commonest lesion, found in up to two-thirds of patients. It is the easiest to repair, particularly when limited to one segment (a single ruptured chorda) and when there is no severe myxomatous involvement. Currently, in centers specializing in heart valve surgery, patients with severe degenerative MR can expect to have their valves repaired with at least 90% probability, very low operative mortality (<1%), greater than 95% freedom from reoperation at 20 years, and normal life expectancy for their age.
When performed by experienced groups, MVr is unrivaled irrespective of the severity of lesions, from simple to complex, which leaflets are involved, and the type of degenerative involvement (myxomatous degeneration or fibroelastic deficiency). Its results should be viewed as the benchmark for other present and future technologies. Nevertheless, continued investment in transcatheter procedures is of the utmost importance to enable development and improved accessibility, particularly for patients who are considered unsuitable for surgery.
Conflicts of interestThe authors have no conflicts of interest to declare.