Pulmonary vein isolation is the cornerstone of atrial fibrillation ablation and is effective for preventing arrhythmias recurrences, especially in patients with paroxysmal atrial fibrillation. During the last years, cryoballoon ablation has emerged as an unquestionable alternative approach to radiofrequency ablation. Many non-randomized and randomized trials have proven undoubtedly that cryoballoon ablation displays similar efficacy and overall safety profile, when compared to radiofrequency ablation for the treatment of patients with drug-refractory paroxysmal atrial fibrillation. These results have been obtained in all types of pulmonary veins anatomical subsets, which confirms that there is no need to select patients according to the latter. The value of cryoablation in the setting of short persistent atrial fibrillation still needs more evidence. Importantly, cryoballoon ablation seems to be less operator-dependent and more reproducible than radiofrequency for the isolation of pulmonary veins.
Atrial fibrillation (AF) is the most common sustained tachyarrhythmia, seen in 1% to 2% of the general population. Pulmonary vein isolation (PVI) is the cornerstone of AF ablation1 and is effective for preventing arrhythmias recurrences, especially in patients with paroxysmal AF. During the last years, cryoballoon (CRYO) ablation has emerged as an unquestionable alternative approach to radiofrequency (RF) ablation and has proven to be at least equivalent for PVI in patients with paroxysmal AF. The recent results of the must FIRE & ICE Trial have definitively closed the debate in this field.2 In addition, the relative simplicity, faster learning curve, and perhaps even more important, the remarkable reproducibility associated with this approach have led to widespread adoption of this technology in clinical practice.3
Clinical evidenceEfficacy of cryoballoon ablation vs. RFFreedom from AF recurrence at 18 months in 75-80% of patients following CRYO ablation of paroxysmal AF using the first-generation balloon has been previously reported.4,5 Several observational single-centre comparisons of CRYO vs. RF ablation in patients with paroxysmal AF have confirmed the non-inferiority of CRYO ablation with regard to efficacy.6–8
A similar efficacy also seems to occur while using the novel generation RF (allowing contact force assessment) and CRYO (second-generation) catheters according to data from single-centre9,10 and multicentre observational studies.11 Results of the first randomized controlled study comparing CRYO vs. RF ablation, the single-centre ‘A Comparison of Isolating the Pulmonary Veins With the Cryoballoon Catheter Versus Radiofrequency Segmental Isolation: a Randomized Controlled Prospective Non-inferiority Trial’ (FreezeAF) have been recently published. FreezeAF included 322 patients with paroxysmal AF who were randomized 1:1 to the first-generation CRYO vs. RF. After a single ablation procedure, 65% of RF-treated patients and 68% of those treated with the CRYO remained in sinus rhythm at 12 months (p<0.001 for non-inferiority).12
A North American multicentre (five centres) observational study compared the success rate of a single procedure of AF ablation using the second-generation CRYO vs. point-by-point non-contact force-sensing RF. In the group of patients with paroxysmal AF (593 treated with CRYO ablation and 320 with RF), a significantly higher number of patients remained free from AF without anti-arrhythmic drugs after CRYO ablation (78.4% Cryoballoon vs. 60.8% RF; log-rank p<0.001).13
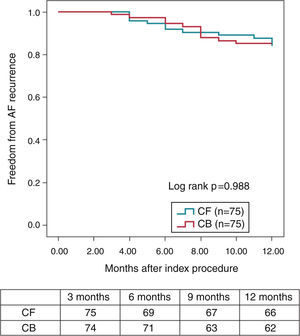
Pulmonary vein isolation is known to be particularly challenging due to the relative difficulty in maintaining a good contact with the tissue all over the encirclement, leading Contact-Force (CF) technology particularly welcome in this setting. Regarding CRYO ablation, it has recently been reported that the new generation CRYO improves the efficiency of the procedure reducing the time to PV isolation, procedural time, and overall success compared with the first-generation balloons.14–16 A non-randomized, prospective trial comparing both technologies suggests that CF real-time assessment using a SmartTouch™ (Biosense, Diamond Bar, CAL) catheter and CRYO ablation using the novel Artic Front Advance™ (Medtronic, Minneapolis, MN) display a very similar procedural efficacy and safety. More importantly, these results also suggest that the mid-term effectiveness profile as regards the 12-months recurrence rate (almost 85% of patients remaining free of AF without antiarrhythmic drugs) is highly similar between both the groups (Figure 1).9
Kaplan–Meier survival curve—proportion of patients free of AF during the 12-month follow-up (3-month blanking period) (with the permission from the authors).9
Last but not least, the recent multicentre European randomized FIRE & ICE trial, has demonstrated that CRYO ablation was non-inferior to RF ablation with respect to efficacy for the treatment of patients with drug-refractory paroxysmal atrial fibrillation.2 These results led to the latest guidelines for using RF or CRYO ablation for the isolation of the pulmonary veins, with the same class and level of recommendation (IIa B).1
Safety of cryoballoon ablation vs. radiofrequency ablationA similar overall safety profile of the two techniques has been suggested by several single-centre observational studies.6–9
In the single-centre, randomized, FreezeAF trial, temporary phrenic nerve palsy and vascular complications occurred more frequently in CRYO ablation (5.8 vs. 0 and 5.1 vs. 1.9%, respectively).12
In the North American Multicentre observational study, a higher rate of complications was also observed with CRYO ablation, driven by an increase in phrenic nerve palsy (7.6 vs. 0%; p<0.001), which was persistent in 1.2% of patients. On the other hand, a trend for more pericardial complications was observed with RF (1.7 vs. 0.6%; p=0.09).13 This trend for less pericardial effusions in patients undergoing cryoablation has also been observed in a meta-analysis by Cheng et al.17 (2.1 vs. 5.5%, OR=0.58, 95% CI 0.30-1.06, p=0.08). A possible explanation for this may be the more uniform distribution of pressure across a higher area of tissue with the CRYO compared with the much smaller and punctiform ablating surface of the tip of the RF catheter which, in the presence of sudden variations and high levels of contact-force, may be more prone to microperforation.
FIRE & ICE also confirmed that there was no significant difference between the two ablation methods with regard to overall safety.2
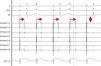
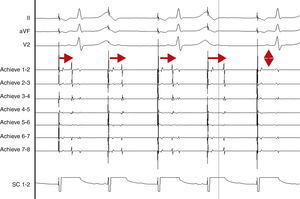
AdvantagesReal-time documentation of PV disconnection by CRYO has been associated with shorter procedure and fluoroscopy times.18 Also, documentation of an early disconnection of the PV has been shown to lead to a higher procedural success rate, with shorter PV isolation times predicting a sustained disconnection.19,20 The feasibility of real-time assessment of PV disconnection in a vast majority of cases helps to better tailor the procedure (the number and duration of cryo-applications) for each patient. This is now easily obtained thanks to the use of the Achieve™ (Medtronic, Minneapolis, MN) catheter, a careful mapping of PV ostia, and some easy-to-do manoeuvers, in about 90% of the PVs (Figure 2). This “real-time electrophysiological approach” has notably improved the CRYO procedure, its reproducibility, and reliability.21
Real-time assessment of the left superior pulmonary vein disconnection while freezing with a 28 mm Artic Front Advance (Medtronic, Minneapolis, MN) balloon. Pacing from the distal coronary sinus with an AV nodal conduction block (Wenckebach phenomenon). Note the progressive increasing of the delay between the left atrial far-field and the pulmonary vein potential (horizontal arrows), leading to a complete disconnection of the vein (vertical arrow).
SC 1-2: distal coronary sinus (pacing); Achieve 1-2… 7-8: bipolar electrograms displayed by the Achieve™ (Medtronic, Minneapolis, MN) circular mapping catheter positioned at the proximal part of the left superior pulmonary vein, just in front of the cryoballoon, while freezing.
Even when the highest values of the maximal PV diameters among persistent AF patients are taken into account (ranging from 20.1 to 22.9 mm), they were considerably less than the critical value of 28 mm, which is the maximal diameter of the CRYO used in everyday practice.22 Due to this area mismatch between balloon and PVs, when this device is positioned against the PV antrum, its cooling distal hemisphere comes in contact not only with the PV antra but also with adjacent atrial myocardial tissue, which seems to be an important bonus of this procedure. Kenigsberg et al. elegantly calculated the area of the ablated cardiac tissue after cryoablation of the PVs by performing a post-cryoablation electro-anatomical voltage map of the left atrium.23 In total, only 27% of the entire left atrial posterior wall surface area remained electrically intact and unablated following cryoablation with the 28-mm CRYO.
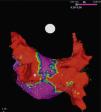
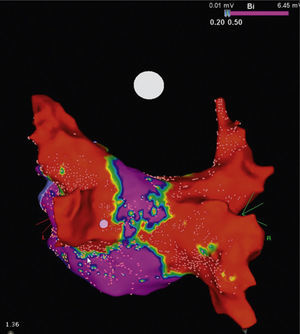
It is to emphasize that although the CRYO conceptually only targets the PVs, it additionally performs considerable electrical debulking of the left atrium, more particularly of the posterior wall (Figure 3).24 This widely circumferential extension of the cooling area may provide collateral benefit by ablating local contributors in AF triggering and maintenance such as ganglionic plexi and rotors, which may have therapeutic implications among patients with persistent AF.25
High density voltage map of the posterior wall after a cryoablation procedure in a persistent AF patient. Note the narrow corridor (purple) remaining between both large scars (red) obtained with the 28 mm Artic Front Advance™ (Medtronic, Minneapolis, MN).24 (Courtesy of Prof. Mario Oliveira, Santa Marta Hospital, Lisboa, Portugal.)
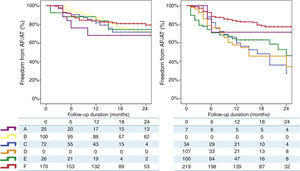
Finally, a non-randomized, multicentre, prospective registry, suggests that, unlike RF, where a marked centre and operator dependence is observed in mid-term results, CRYO ablation seems to perform equally well, with a less-pronounced impact of centre or operator experience.3 A total of 860 consecutive patients undergoing a first ablation procedure for paroxysmal AF (467 treated with RF and 393 treated with CRYO) were selected from a prospective multicentre survey of AF ablation (FrenchAF). Radiofrequency and CRYO were compared regarding mid-term efficacy and safety. During a median follow-up of 14 months (interquartile range 8-23), patients treated with CRYO displayed similar rates of freedom from atrial arrhythmia relapse in centres performing this technique (68-80% at 18 months). However, in centres performing RF, a greater heterogeneity in procedural results was observed (46-79% were free from atrial arrhythmia relapse at 18 months) (Figure 4). On multivariate analysis, CRYO (HR=0.47, 95% CI 0.35-0.65, p<0.001) and annual AF ablation caseload (HR=0.87 per every 100 AF ablation procedures per year; 95% CI 0.80-0.96, p=0.003) were independent predictors of procedural success. These real-world data suggest that CRYO ablation seems to be less operator-dependent and more reproducible than RF in the setting of paroxysmal AF ablation.3
Overview of procedural results and freedom from AF in patients treated with Cryoballoon (left) and RF (right) (with the permission from the authors).3
Legend: A, B, C, D, E and F: different volume centres; Cryoballoon: cryoballoon ablation; RF: radiofrequency ablation.
Although CRYO characteristics and success rates are improved in the latest studies including persistent AF patients,26,27 a relatively higher rate of recurrence in this setting, might raise questions regarding the efficacy of one index procedure for long-term clinical success. The same limitation can also be reported for RF. Importantly, this higher rate of recurrence in persistent AF might not be solely attributed to conduction recovery at PV ostium. This was also shown in recent studies, raising concerns about the role of non-PVI sources providing the mechanism for recurrence.25,27
On another hand, contradictory results have been published so far regarding the influence of anatomy on outcomes after paroxysmal AF ablation using CRYO ablation. Kubala et al.28 have reported a higher proportion of patients free from AF relapse in those with a normal PV pattern.
However, CRYO ablation data in this study were not compared with RF data, and only first-generation (Arctic Front™, Medtronic, Minneapolis, MN) CRYO were used. Potential reasons suggested by the authors were: the difficulty of correctly evaluating the isolation compared with normal PVs, the difficulties in manipulating catheters with a conventional fixed size and shape in unusual vein anatomies, and the abnormal localization of AF foci in this population. At around the same time, Defaye et al. published data concerning 220 patients and found no difference in mid-term outcomes when in the presence of a common ostium.29 In a substudy from the Sustained Treatment Of Paroxysmal Atrial Fibrillation trial, the presence of anatomical PV variants was also not significantly associated with early or late recurrences in paroxysmal AF patients treated using cryoballoon ablation. Consistent with these findings, Ferrero-de Loma-Osorio et al. did not find that the presence of a common left PV ostium was associated with a lower rate of acute PV isolation or worse mid-terms results.30 Finally, Neumann et al.31 reported no influence of the presence of a left common ostium on long-term results. However, all of these studies had low numbers of abnormal PVs patients and therefore results must be interpreted with caution. Knecht et al.32 suggested that the presence of a sharp carina between the left superior and left inferior PV and a sharp left lateral ridge between the left appendage and the left superior PV could predict acute and mid-term procedural failure after CRYO ablation. They also concluded that the presence of a supernumerary vein did not seem to play a role in mid-term results.
Other authors compared the results of CRYO and RF ablation in anatomical variants of PV distribution, and they concluded that a left common ostium should not be considered a contraindication to CRYO ablation.33 Many reasons support this affirmation. First, the use of the largest (28 mm) balloon in all patients is now an accepted policy for the majority of practitioners, resulting in a wider antral lesion. Second, the sheath supporting the balloon and the balloon itself are deflectable, allowing the operator to engage the different branches of the vein separately and thus overcome the difficulties derived from the presence of a large common ostium.34 Third, the use of the second-generation CRYO together with the Achieve™ (Medtronic, Minneapolis, MN) catheter also seems to play a determinant role. This balloon improves cooling capabilities by increasing the number of refrigerant injectors, resulting in a bigger and more homogeneous cooling of the balloon surface. In addition, the Achieve™ (Medtronic, Minneapolis, MN) catheter increases the stability of the balloon and allows the real-time assessment of PV disconnection in most cases.21,35
For right supernumerary PVs, data in the literature are scarce. For many operators, the presence of right supernumerary vein(s) is an exclusion criterion for CRYO ablation. Preliminary data from a very small sample of patients (without an RF control group) suggest that right upper PV diameter may be an independent predictor of relapse.36 Another experience, in 47 patients with supernumerary right vein(s), reported that the presence of such an anatomical variant does not influence the mid-term results of the procedure.33 The use of a 28-mm balloon in these patients allows a large antral PVI and probably encompasses supernumerary veins. These findings illustrate that CRYO ablation performs similarly to RF in paroxysmal AF patients in terms of mid-term results and in all types of PV anatomical subsets. This suggests that patient selection based on anatomical criteria is not mandatory for patients undergoing CRYO ablation for paroxysmal AF.33
In summaryCryoballoon ablation displays similar efficacy and overall safety profile, when compared to radiofrequency ablation for the treatment of patients with drug-refractory paroxysmal atrial fibrillation, in any anatomical configuration, without the need to select patients according to the latter. Its value in the setting of short persistent atrial fibrillation needs more evidence. Importantly, cryoballoon ablation seems to be less operator-dependent and more reproducible than radiofrequency for the isolation of pulmonary veins.
Disclosures and conflicts of interestBoveda S receives consulting fees from Medtronic, Boston Scientific and Livanova.