In the last years, atrial fibrosis was shown to be an independent predictor of procedural failure in patients with paroxysmal and persistent atrial fibrillation. Ablation strategies have been developed to improve the outcome of catheter ablation by targeting detected areas of fibrosis, based either on endocardial voltage mapping or cardiac magnetic resonance. Box isolation of fibrotic areas (BIFA) is a new and promising patient-tailored ablation strategy for atrial fibrillation patients targeting substantial fibrotic areas by circumferential isolation of left atrial fibrosis.
Circumferential pulmonary vein isolation (PVI) is the cornerstone for catheter ablation of patients with paroxysmal atrial fibrillation (AF). In a significant amount of patients with non-paroxysmal AF, however, PVI alone seems non-sufficient with lower long-term success despite improved technologies achieving more durable lesions.1 In the last years, atrial fibrosis was shown to be an independent predictor of procedural failure with an increased risk of arrhythmia recurrence after ablation.2–4 There is more and more evidence that atrial fibrosis, being the fundamental histo-pathological finding in AF patients, in many patients is a result of fibrotic atrial cardiomyopathy (FACM), a specific and primary disease with variable expression (mild, moderate or severe fibrosis) and a variable clinical presentation (sinus node disease, atrial fibrillation, atrial tachycardias).5,6 Detecting left atrial fibrosis by electroanatomic voltage mapping (EAVM) or magnetic resonance imaging (MRI) and targeting the fibrotic substrate by catheter ablation seems to improve ablation outcome compared to previous catheter ablation strategies for patients with persistent AF.
Box isolation of fibrotic areas – the ablation strategyAt the beginning of the procedure, a detailed EAVM is performed during sinus rhythm to visualize the individual extent and localization of the left atrial fibrosis. All points are acquired point-by-point using the ablation catheter (Smart-Touch, Biosense Webster, Diamond Bar, CA, USA) to ensure adequate catheter tissue contact by contact force (5-15 g). Only points during stable sinus rhythm are acquired (no points during or immediately after atrial salves or premature atrial beats). Depending on the left atrial size and substrate, 100-200 voltage points are acquired with an interpolation threshold of ≤10 mm to ensure adequate point distribution. A denser sampling is performed at border-zones of substantial fibrotic areas to delineate the extent of fibrosis for subsequent ablation. A computer tomography 3D reconstruction of the left atrium is merged with the EAVM shell. The 3D mapping system (Carto 3, Biosense Webster, Diamond Bar CA) automatically measures peak-to-peak bipolar electrogram amplitude. All mapping points are manually reviewed for correct annotation. Substantial fibrotic areas are defined as an area of ≥3 adjacent voltage points with a bipolar peak-to-peak voltage amplitude <0.5 mV.
In patients with AF at the beginning of the procedure, external cardioversion is performed, to restore sinus rhythm. When cardioversion fails to restore a stable sinus rhythm, circumferential isolation of the ipsilateral pulmonary veins is performed as the first step, followed by cardioversion and EAVM during sinus rhythm. After PVI, circumferential isolation of substantial fibrotic areas is performed. The circumferential ablation lines are, in general, connected to the PV ablation lines to avoid potential proarrhythmic effects of small channels. Ablation endpoint is the complete isolation of both, the pulmonary veins and the left atrial fibrotic areas, confirmed by a circular mapping catheter or with the ablation catheter.
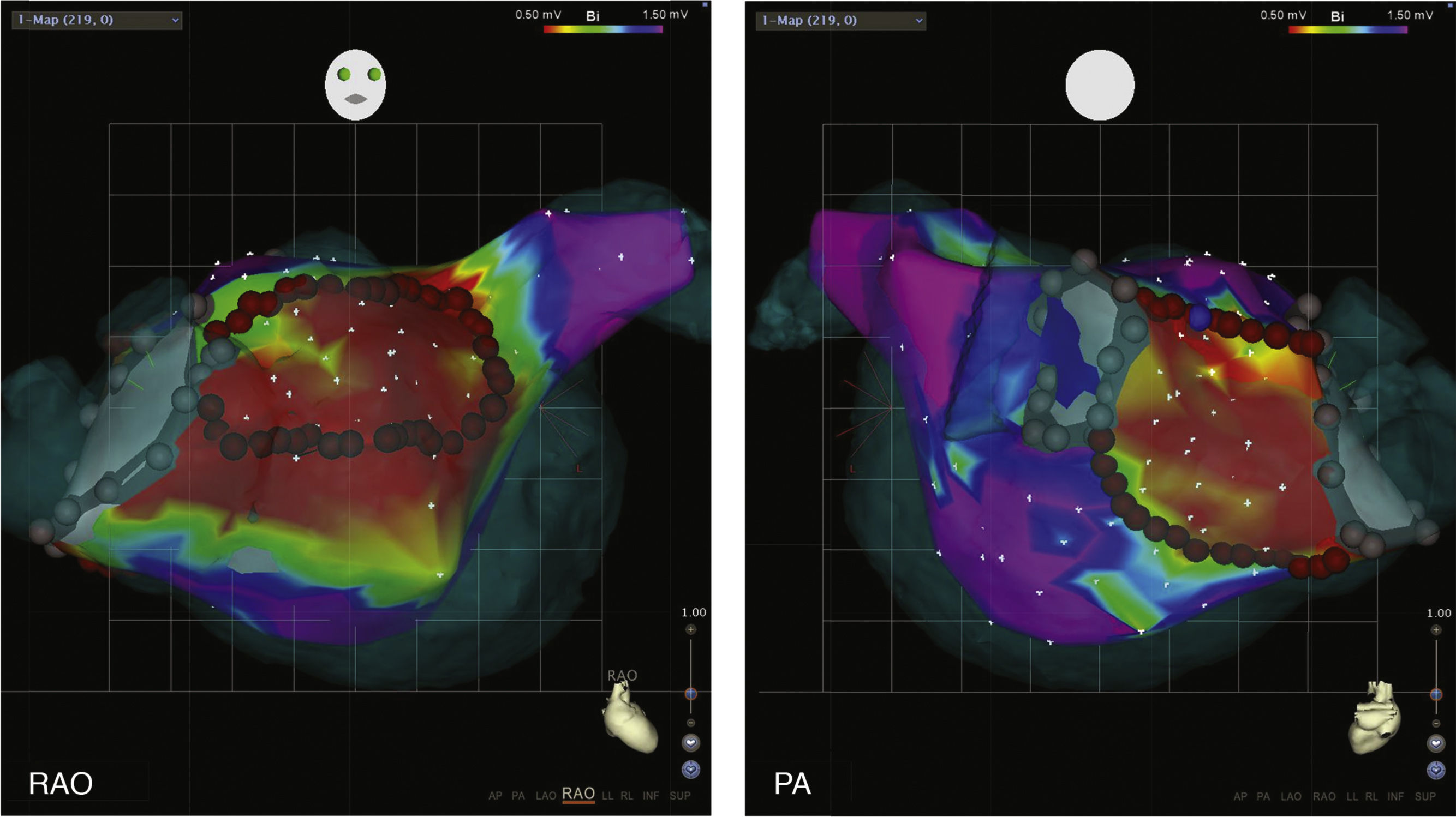
The concept of Box Isolation of Fibrotic Areas was first tested and validated by our group in re-do procedures patients with paroxysmal AF who presented with recurrent AF despite durable PVI.2,3 Left atrial fibrosis was detected by EAVM in all patients (Figure 1). In 9/10 patients, substantial fibrotic areas could be identified and in one patient a confluent area of moderately reduced voltage with fragmented electrograms as an additional surrogate for fibrosis. After a mean follow-up of 20±13 months, 9/10 patients remained in sinus rhythm after BIFA ablation.2,3
Box isolation of fibrotic areas (BIFA).
Box isolation of fibrotic areas (BIFA) in a patient with recurrent atrial fibrillation despite durable pulmonary vein isolation, according to individual localization and extent of substantial left atrial fibrosis. Color coding of voltage maps: red <0.5 mV and purple >1.5 mV. Grey points represent points with no detectable electrogram at previous ablation lines, indicating pulmonary vein isolation. PA: posterior-anterior projection; RAO: right anterior oblique projection.
Subsequently, BIFA was extended to initial procedures in patients with persistent AF.3 In 18/31 patients (58%), substantial fibrotic areas were identified and box isolation in addition to PVI was performed. In the remaining 13 patients (42%), no fibrosis was detected and ablation was limited to PVI without any additional substrate modification. Of those patients with left atrial fibrosis, 15/18 (83%) stayed in sinus rhythm after 1.06 procedures during a follow-up of 11±6 months. Eleven of 13 patients (85%) without left atrial fibrosis were in sinus rhythm with 1.15 procedures/patient after a mean follow-up of 14±8 months. Overall, 26/31 (84%) patients with non-paroxysmal AF had no recurrence of AF or atrial tachycardia with 1.13 procedures/patient during a follow-up of 12±7 months.3
In a recent analysis by our group, 92 AF patients (58 persistent/34 paroxysmal), with substantial left atrial fibrosis underwent PVI plus BIFA, and 49 patients without fibrosis only underwent PVI and served as a control group.7 Single and multiple procedure success with 1.2 procedures/patients during a follow-up of 16±8 months was 69% and 83%, respectively, without any significant difference in patients with paroxysmal AF compared to patients with non-paroxysmal AF (86.9% vs 80.3%, p=0.51). No significant difference was observed between patients with substantial fibrosis limited to only one area undergoing the BIFA strategy in addition to PVI, compared to the control group without fibrosis undergoing PVI alone (multiple procedure success rate: 93% vs. 94% during a 12-month FU). The difference in outcome between patients with left atrial fibrosis who underwent BIFA ablation compared to patients without left atrial fibrosis was mainly driven by those patients with a substantial and diffuse fibrotic substrate (FACM grade 4). In this relatively small cohort of AF patients, a clear BIFA concept may not be applicable and may not improve procedural outcome due to massive fibrosis.
In general, the BIFA ablation strategy is applicable in patients with well-demarcated fibrotic areas and as such, it is a promising new patient-tailored substrate modification approach in addition to PV isolation. Our results also outline that additional substrate modification in patients without substantial atrial fibrosis may not be necessary and PVI is enough for adequate rhythm control even in patients with persistent AF.
Several other groups confirmed our results with an improved ablation outcome by EAVM guided substrate modification following PVI.8–11 However, all studies differ in terms of ablation strategy, voltage-cut-offs defining fibrotic areas, and procedural endpoint from our BIFA approach.
Yamaguchi et al. reported a single procedure success rate of 72% after PVI and voltage based catheter ablation in patients with persistent AF, whereas 79% of patients with persistent AF but without detected fibrosis undergoing PVI alone were in sinus rhythm after 18±7 months.10 Procedural endpoint of this approach with “homogenization” of areas with low voltage was defractionation and/or electrogram voltage reduction of >50%. Comparing this homogenization approach with BIFA, box isolation may have the benefit of less burning, especially in atria with large and substantial fibrotic areas. Furthermore, voltage reduction is not a typical sign of complete isolation in our experience but more for incomplete isolation. For the BIFA strategy, no discernible electrogram other than farfield or noise inside the box is defined as the procedural endpoint.
Jadidi et al. demonstrated that ablation targeting sites with distinct activation characteristics within fibrotic areas or at its border zones in addition to PVI was more effective than PVI alone for persistent AF patients.11 After a median follow-up of 13 months, single arrhythmia freedom after PVI and selective LVA ablation was significantly higher than in the matched control group undergoing PVI alone (69% vs. 47%, p<0.009). Interestingly, AF termination sites were within fibrotic areas in 80% and at its border zones in 20%. This is consistent with our experience, where AT termination sites are usually located in areas of severe fibrosis with AT termination during box isolation of fibrotic areas.
Recently, Yagishita et al. presented a similar long-term ablation outcome in patients with substantial fibrosis undergoing voltage guided substrate modification following PVI compared to patients without substantial fibrosis undergoing PVI alone.12 In 70% of patients with persistent AF, fibrotic areas were detected, and after voltage-guided substrate ablation 74% of patients had no arrhythmia recurrence during a median follow-up of 3.1 years. In contrast to our strategy, voltage mapping was performed during AF. A linear correlation between left atrial bipolar voltage in SR and in AF was reported.13 However, heterogeneous activation and temporal instability is causing a marked variability of the voltage amplitude, compared to voltage mapping during sinus rhythm.
ConclusionDespite these differences in substrate modification strategies, targeting substantial fibrotic areas has been shown to be an effective tool for catheter ablation in AF. Individual patient tailored ablation of the fibrotic substrate, triggering and maintaining AF, improves ablation results, independent from the “phenotype” paroxysmal versus non-paroxysmal AF.
Conflicts of interestH.K.: Consultant of Biosense Webster, Consultant/equity interest of Kardium.