Pulmonary vein isolation (PVI) is central to ablation approaches for atrial fibrillation (AF), yet many patients still have arrhythmia recurrence after one or more procedures despite the latest technology for PVI. Ablation of rotational or focal sources for AF, which lie outside the pulmonary veins in many patients, is a practical approach that has been shown to improve success by many groups. Localized sources lie in atrial regions shown mechanistically to sustain AF in optical mapping and clinical studies of human AF, as well as computational and animal studies. Because they arise in localized atrial regions, AF sources may explain central paradoxes in clinical practice – such as how limited ablation in patient specific sites can terminate persistent AF yet extensive anatomical ablation at stereotypical locations, which should extinguish disordered waves, does not improve success in clinical trials. Ongoing studies may help to resolve many controversies in the field of rotational sources for AF. Studies now verify rotational activation by multiple mapping approaches in the same patients, at sites where ablation terminates persistent AF. However, these studies also show that certain mapping methods are less effective for detecting AF sources than others. It is also recognized that the success of AF source ablation is technique dependent. This review article provides a mechanistic and clinical rationale to ablate localized sources (rotational and focal), and describes successful techniques for their ablation as well as pitfalls to avoid. We hope that this review will serve as a platform for future improvements in the patient-tailored ablation for complex arrhythmias.
Ablation is increasingly used to treat atrial fibrillation (AF), yet the 1-2 year success rate of pulmonary vein isolation (PVI) remains 40-50% for persistent AF1,2 and 50-65% for paroxysmal AF.3–5 Studies of the often extensive ablation of lines, empiric electrogram targets and particularly the posterior left atrium often have not improved success versus PVI alone in multicenter trials.1,2,6
Targeting AF sources has gained much attention in recent years. In the source model, AF is sustained by rotational (rotor) or focal sources in localized patient-specific regions of either atria. This model is now supported by wide evidence in many patients ranging from optical mapping of human AF,7 multicenter (non-randomized) clinical trials of AF rotor ablation,8–13 optical mapping of animal AF,14 and mathematical analyses.15 Moreover, this model can reconcile the paradox that limited ablation can terminate persistent AF in some patients,8,16,17 while extensive (untargeted) ablation of left atrial regions can be ineffective in others.1,2,18
The source model may contradict the multiwavelet hypothesis, in which AF is caused by disordered waves alone,19,20 although ongoing studies on the interaction between organized sources and disordered waves may reconcile this. A central tenet of the ‘pure’ disordered model is that extensive ablation limits the critical mass for wave propagation and will increase success, yet this is contradicted by multicenter trials1,2,6 with suboptimal results even in some surgical studies21 compared to original reports.
This review provides an overview of the science for localized drivers of human AF, and technical factors explaining why they may be revealed by some but not all mapping approaches. In particular, we focus on potential explanations for why some clinical ablation studies have been disappointing despite promising results at many independent centers. We hope to provide a mechanistic/clinical foundation to help reconcile debates in this field.
Initiation of human atrial fibrillationHuman arrhythmias have dynamic and static mechanisms.22 In AF, triggers such as ectopic beats,23 bursts of atrial tachycardia24 or varying autonomic balance25,26 may on occasion initiate AF as a dynamic process, despite a relatively static atrial architecture and fibrosis.27
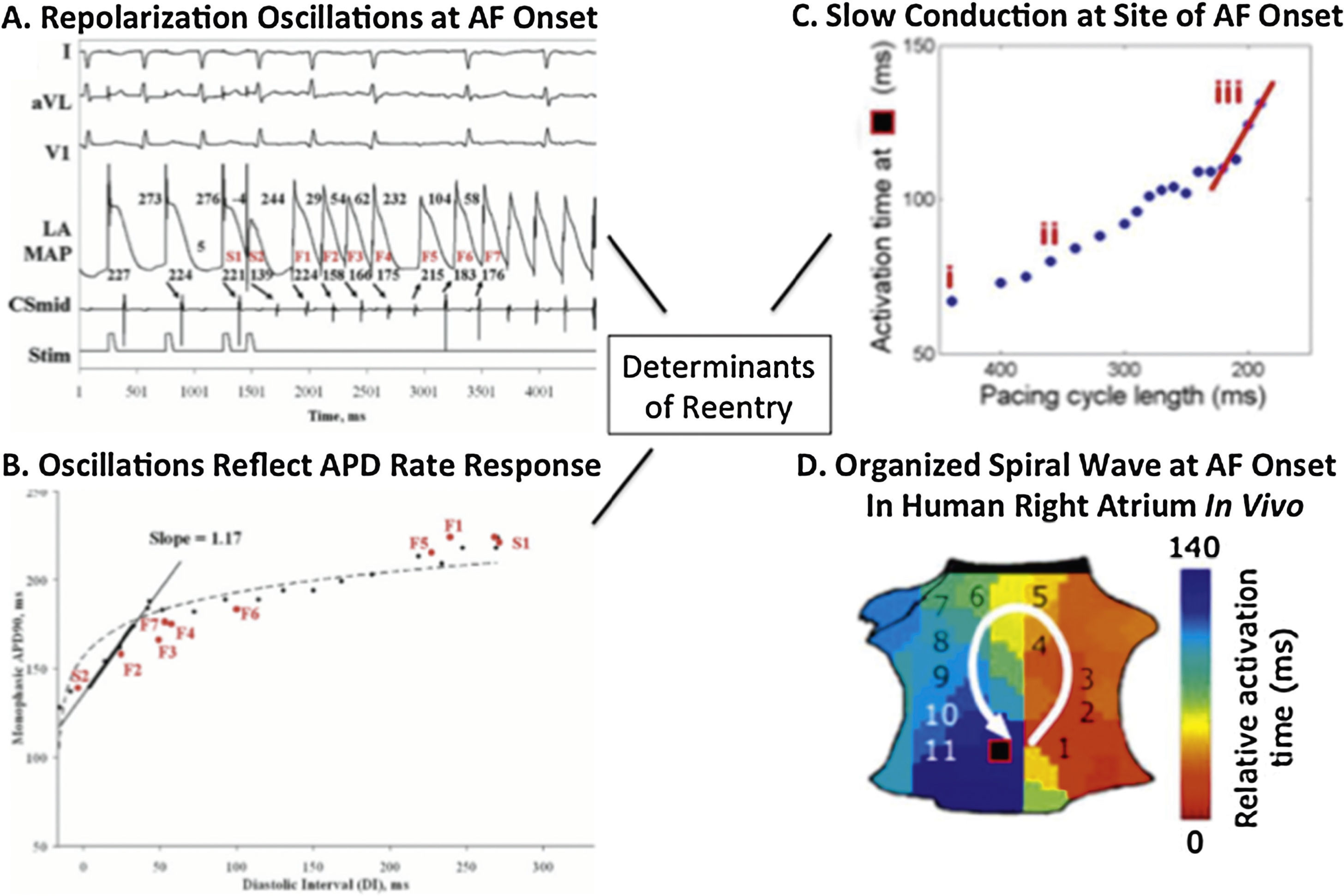
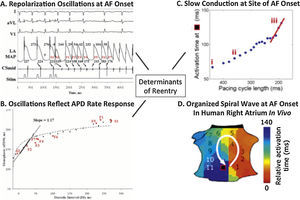
Dynamics in the physiology of atrial repolarization and conduction can explain how triggers initiate AF. In Figure 1A, an ectopic beat produces dramatic oscillations of human left atrial action potential duration (APD),24,28,29 because the graph relating APD to diastolic interval (DI, time between beats; Figure 1B)30 is steep, so that an early beat (short DI) drastically shortens APD, lengthening subsequent DI, producing APD alternans and wavebreak. Figure 1C shows that triggers, such as from the PVs, may also abruptly slow human atrial conduction to facilitate reentry and AF (Figure 1D).31 These dynamics likely interact with atrial anatomy32 and/or fibrosis,33 explaining why sites of spiral wave reentry may initiate at spatially conserved sites for diverse triggers.
Initiating mechanisms for human atrial fibrillation. (A) Electrograms of ectopy (S2) initiating AF. (B) Steep relationship of atrial repolarization to preceding interbeat interval (APD vs DI) enables S2 to produce repolarization oscillations. (C) Conduction also shows drastic slowing imminently preceding AF onset (iii, red slope). (D) Conduction slowing and dispersion of repolarization cause formation of a counter-clockwise spiral on the atrial shell, that initiated AF and was conserved for multiple AF initiations and diverse triggers in this patient.
Once AF is initiated by triggers from pulmonary veins23 or other sites, two central hypotheses may explain how disorganized wavefronts in AF sustain. In the multi-wavelet hypothesis,19 disorganized activity generates new wavelets in a stochastic fashion, where no specific atrial region plays a crucial role in the AF maintenance. This hypothesis therefore precludes “special” regions of the atria, such that structural elements e.g. fibrosis distribution are not critical to the maintenance of AF. A second, alternative, mechanism is that “special” regions of the atria do indeed exist and act as functional sources, manifest electrically in the form of spiral waves or focal activation patterns. These organized sources generate wavefronts that break down (“fibrillatory conduction”) to explain the disorder of AF.
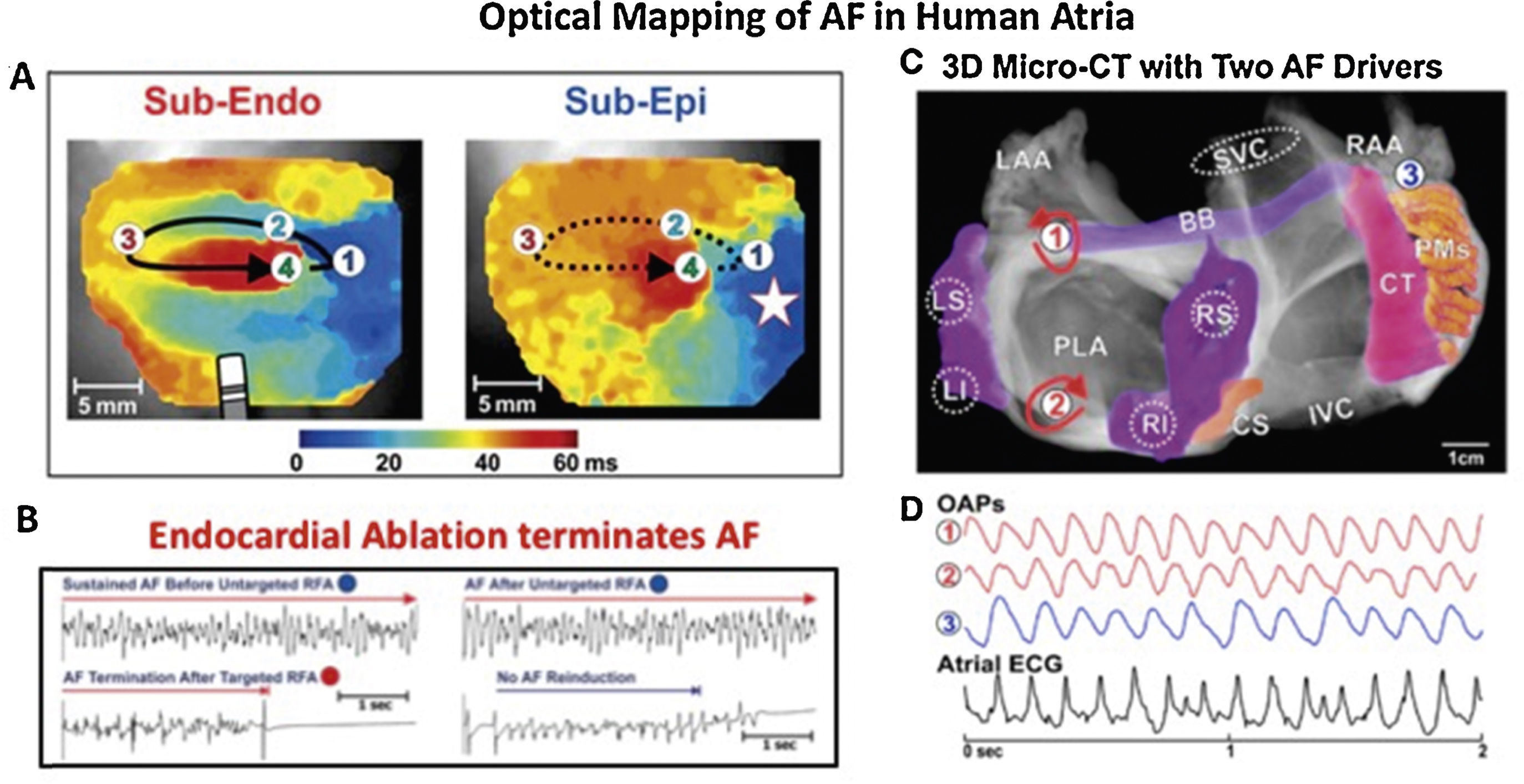
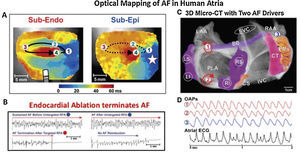
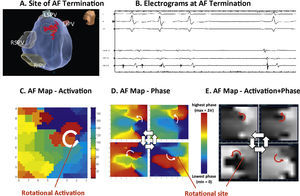
Some of the most important evidence for localized drivers of human AF comes from optical mapping. Figure 2 shows results from the gold standard of optical mapping of human AF.7,34 Rotational drivers are seen (Figure 2A) where ablation terminated AF to sinus rhythm (Figure 2B) and, in another specimen, 2 concurrent AF sources are seen in left atrium (Figure 2C). Sources hence lay in either atrium in these diseased atria, stabilized by micro-reentry around fibrotic and slowly conducting regions, which are less reported in animal models. One important methodological issue arising from this work, that we will revisit later, is that action potentials in AF may be quite regular (Figure 2D), yet traditional electrograms show additional deflections in AF (“noise”, “far-field activation”) which will reduce accuracy for mapping.
Human atrial fibrillation sustained by stable rotational sources on optical mapping.7,34 (A) Rotational AF source in right atrium, stable on endocardium yet transient on epicardium. (B) Ablation at AF source terminates AF.7 (C) Concurrent AF sources near and remote from PVs, anchored to micro-fibrosis and fiber architecture. (D) Electrograms in AF, unlike regular optical potentials, comprise noise including far-field signals.34
Despite data in favor of rotational or focal drivers for human AF,8,10 there is an active debate on this issue. First, historical AF mapping shows only disorganized waves with no driving regions,35 albeit in patients rarely referred for ablation (with permanent AF at non-arrhythmia surgery). Second, organized drivers by frequency analysis36 may be unstable by activation37 or phase38–40 maps. This may reflect mapping of the epicardium in these studies, where drivers are less stable on optical maps.7,34 Third, AF-driver ablation outcomes are disappointing at some centers41–43 (Table 1 summarizes PVI and driver ablation studies). Mapping differences will alter reported AF mechanisms, and Rudy et al. show that combined activation with phase may be optimal in AF.44 The method used by our group, Focal Impulse and Rotor Modulation (FIRM), uses such a combined activation and phase approach.45,46 However, it is unresolved to what extent conflicting studies reflect mapping methods, patient selection, species specificities or other differences.
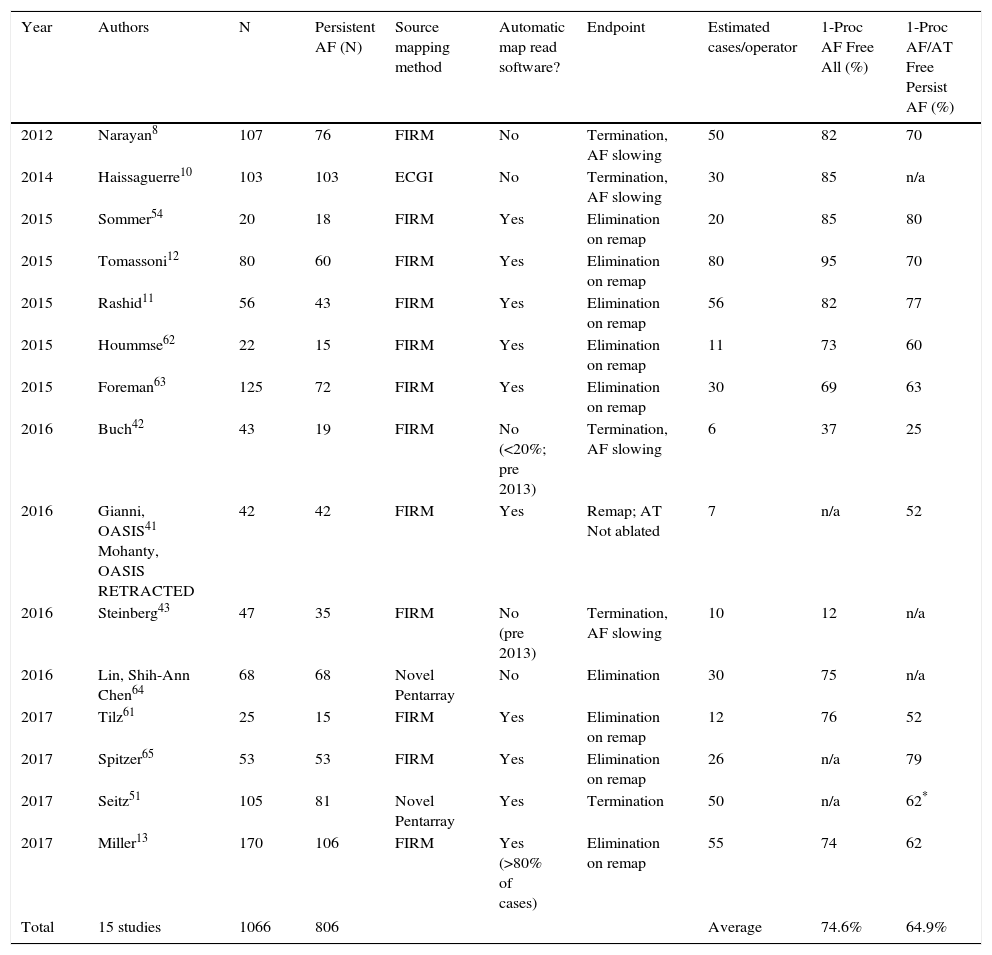
Single procedure success of AF source plus PVI ablation. Average results weighted by number of patients per study.
| Year | Authors | N | Persistent AF (N) | Source mapping method | Automatic map read software? | Endpoint | Estimated cases/operator | 1-Proc AF Free All (%) | 1-Proc AF/AT Free Persist AF (%) |
|---|---|---|---|---|---|---|---|---|---|
| 2012 | Narayan8 | 107 | 76 | FIRM | No | Termination, AF slowing | 50 | 82 | 70 |
| 2014 | Haissaguerre10 | 103 | 103 | ECGI | No | Termination, AF slowing | 30 | 85 | n/a |
| 2015 | Sommer54 | 20 | 18 | FIRM | Yes | Elimination on remap | 20 | 85 | 80 |
| 2015 | Tomassoni12 | 80 | 60 | FIRM | Yes | Elimination on remap | 80 | 95 | 70 |
| 2015 | Rashid11 | 56 | 43 | FIRM | Yes | Elimination on remap | 56 | 82 | 77 |
| 2015 | Hoummse62 | 22 | 15 | FIRM | Yes | Elimination on remap | 11 | 73 | 60 |
| 2015 | Foreman63 | 125 | 72 | FIRM | Yes | Elimination on remap | 30 | 69 | 63 |
| 2016 | Buch42 | 43 | 19 | FIRM | No (<20%; pre 2013) | Termination, AF slowing | 6 | 37 | 25 |
| 2016 | Gianni, OASIS41 Mohanty, OASIS RETRACTED | 42 | 42 | FIRM | Yes | Remap; AT Not ablated | 7 | n/a | 52 |
| 2016 | Steinberg43 | 47 | 35 | FIRM | No (pre 2013) | Termination, AF slowing | 10 | 12 | n/a |
| 2016 | Lin, Shih-Ann Chen64 | 68 | 68 | Novel Pentarray | No | Elimination | 30 | 75 | n/a |
| 2017 | Tilz61 | 25 | 15 | FIRM | Yes | Elimination on remap | 12 | 76 | 52 |
| 2017 | Spitzer65 | 53 | 53 | FIRM | Yes | Elimination on remap | 26 | n/a | 79 |
| 2017 | Seitz51 | 105 | 81 | Novel Pentarray | Yes | Termination | 50 | n/a | 62* |
| 2017 | Miller13 | 170 | 106 | FIRM | Yes (>80% of cases) | Elimination on remap | 55 | 74 | 62 |
| Total | 15 studies | 1066 | 806 | Average | 74.6% | 64.9% | |||
Since long-term ablation success varies dramatically between centers even for PVI,47 reconciling differences in AF mapping requires other study designs. On the basis that sites where ablation terminates persistent AF may have mechanistic relevance, we are systematically comparing maps created by different techniques for analyzing the same raw electrographic data in cases of clear termination of persistent AF by ablation in the ongoing COMPARE-AF study.
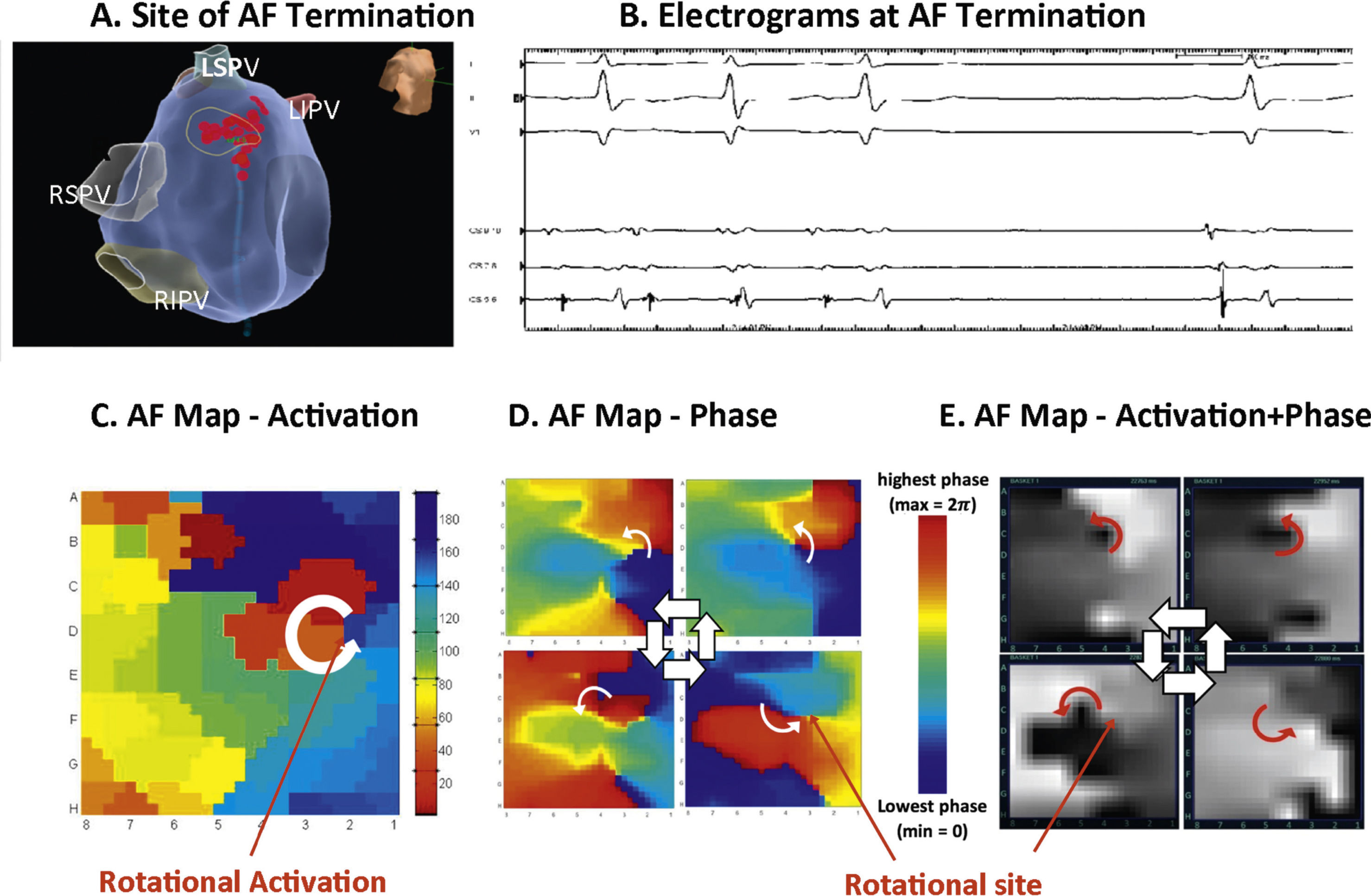
Figure 3 shows a patient in whom ablation outside the PVs before PVI terminated persistent AF to sinus rhythm. In panels C-E, detailed analysis of raw AF electrograms prior to ablation revealed sustained rotational activation by 3 methods.48 Of these methods, traditional activation maps of AF electrograms may be confused by spurious deflections, perhaps reflecting far field (e.g. Figure 2, compared to optical maps), and often showed only disorder at AF termination sites where phase and combined phase/activation maps showed rotations.
Rotational drivers for AF by multiple mapping methods. In a 67 year old woman, ablation (A) at the left atrial roof prior to PVI (B) terminates persistent AF to sinus rhythm. (C) Traditional activation mapping, (D) phase mapping using published methods38 and (E) Focal Impulse and Rotor Mapping (FIRM) each confirmed sustained rotations at the site where ablation terminated AF. Modified from references 66 and 48.
We reported an early series of such patients,48 which was the inspiration for the international COMPARE-AF study (NCT02997254) from which mapping data and code are being made available online to accelerate developments in AF mapping.
Reconciling heterogeneity in the success of source-guided ablationInitial reports of mapping-guided ablation of AF drivers were positive, using endocardial mapping by FIRM in 2011-12,8,49 body-surface mapping,10 novel mapping by Shih-Ann Chen et al.,50 Seitz et al.51 and other centers52,53 (Table 1). However, while independent centers continue to report positive results,11,12,54,55 outcomes are disappointing by some operators41–43 (Table 1).
Table 1 details the heterogeneity in outcomes from map-guided AF source ablation across centers. Notably, this does not appear to stratify by patient comorbidity or disease severity. For instance, Sommer, Hindricks et al.54 and Miller et al.9,13 reported 70-80% maintenance of sinus rhythm by FIRM-ablation in populations that included challenging patients with prior failed procedures and long-standing persistent AF; as did Tomassoni,12 Rashid11 and others. Conversely, Buch, Shivkumar et al.42 report 21% success and Steinberg et al. report 12% success43 in patients even with paroxysmal AF despite also performing PVI. It is difficult to reconcile these results based primarily on disease severity.
Much of the heterogeneity in results between centers may thus reflect technical differences. It is noted that reading AF rotational/focal source maps and/or guiding ablation accordingly are novel skills and not learned as part of traditional PVI. Indeed, in Table 1 lower success was seen at centers with fewer cases per operator, and in patients studied early (pre-2013) when no automatic tools were available to interpret maps to assist the operator. Although follow-up duration is typically a confounder, positive studies in Table 1 are reported at 1-2 years, 2 years12 and 3 years49 by separate groups.
Areas for technical improvement in source-guided ablation of AFThree core components are required for effective source-guided ablation: effective broad-area mapping of both atria, precise identification of sources to target, and ensuring full elimination of target areas. Each of these components also requires urgent technical improvement, since they may explain much of the heterogeneity in outcomes between centers.
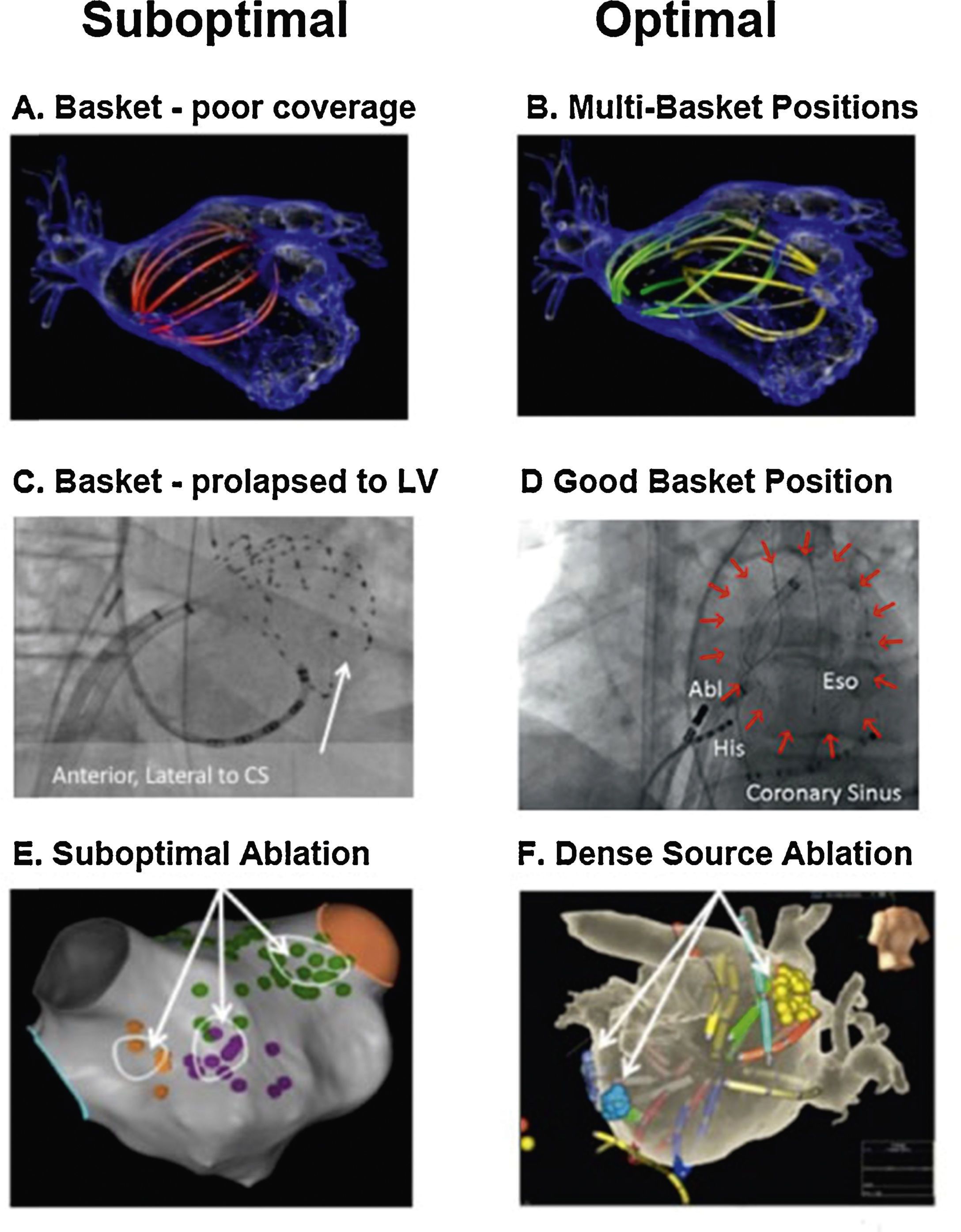
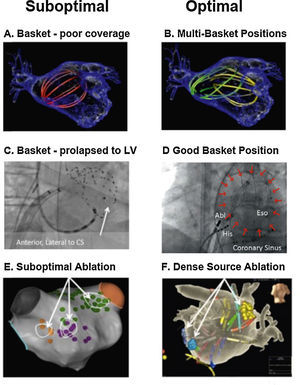
Our approach to widely map the atria is to use multipolar basket catheters to analyze many wavefronts at the same time.45Figure 4 shows optimal and suboptimal basket placement. Various basket sizes and types are available from an increasing number of vendors. We select the most appropriate basket size based on atrial size from intracardiac echocardiography or computed tomography. Since the atrium is not a spherical structure, the basket may have to be moved sequentially for optimal mapping. Figure 4A shows a single suboptimal left atrial basket position, which does not contact the walls (noted by its spherical shape). Figure 4B shows successive repositioning in this case to cover most of the atria in 2-3 epochs, showing spline deformation i.e. good contact. Figure 4C shows that suboptimal basket positioning may not be recognized.56Figure 4D shows careful basket positioning at a high-volume center in a recent large series.57
Reported pearls and pitfalls in AF source ablation. (A) Suboptimal basket position in left atrium. (B) Multi-Position Basket Mapping, the method of choice in large atria. (C) Suboptimal basket in a disappointing report, consistent with inadvertent LV prolapse – conical shape, anterolateral to coronary sinus, ventricular electrograms56; (D) good basket position covering atrium well; (E) sparse lesions over source area in disappointing series41,67; (F) dense source area lesions in a more successful series.54
Theoretically, baskets can resolve 1-2 cm diameter reentry circuits in human AF as predicted by Allessie et al.58 and confirmed in human optical maps (Figure 2A).7 Conversely, if electrodes are too closely spaced, calculated wave propagation may fall within measurement error. For instance, for atrial conduction velocity in AF patients of 40 cm/sec,31,59 reported errors in assigning onset time in AF (≈5-10 ms) translate to a distance of 2-4 mm (=40×0.005 to 40×0.010). Closer electrode separation than this will be attempting to identify circuits within measurement noise, and will have less confidence. A related issue is that closely-spaced electrode arrays typically cover small distances simultaneously, which may also miss the 1-2 cm diameter circuits in human AF noted in optical maps.7,34
Successful source ablation should fully cover the affected source areas, and contact force sensing catheters may help in this regard. Incomplete coverage may explain lower success rates in some source ablation studies. For instance, Figure 4E (Gianni et al.41 in patients from OASIS) shows sparse lesions that may not have eliminated sources in the long-term even if AF terminates acutely. Conversely, in the successful series in Figure 4F, ablation lesions densely cover the affected atrial regions.
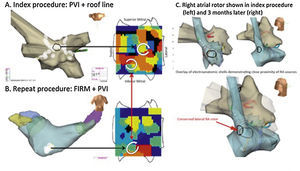
As shown in Figure 5, incompletely eliminated source regions may cause recurrent post-ablation AF or AT. Thus, it is critical to ablate the entire affected region, although the science on how much atrium to ablate has yet to be fully established.
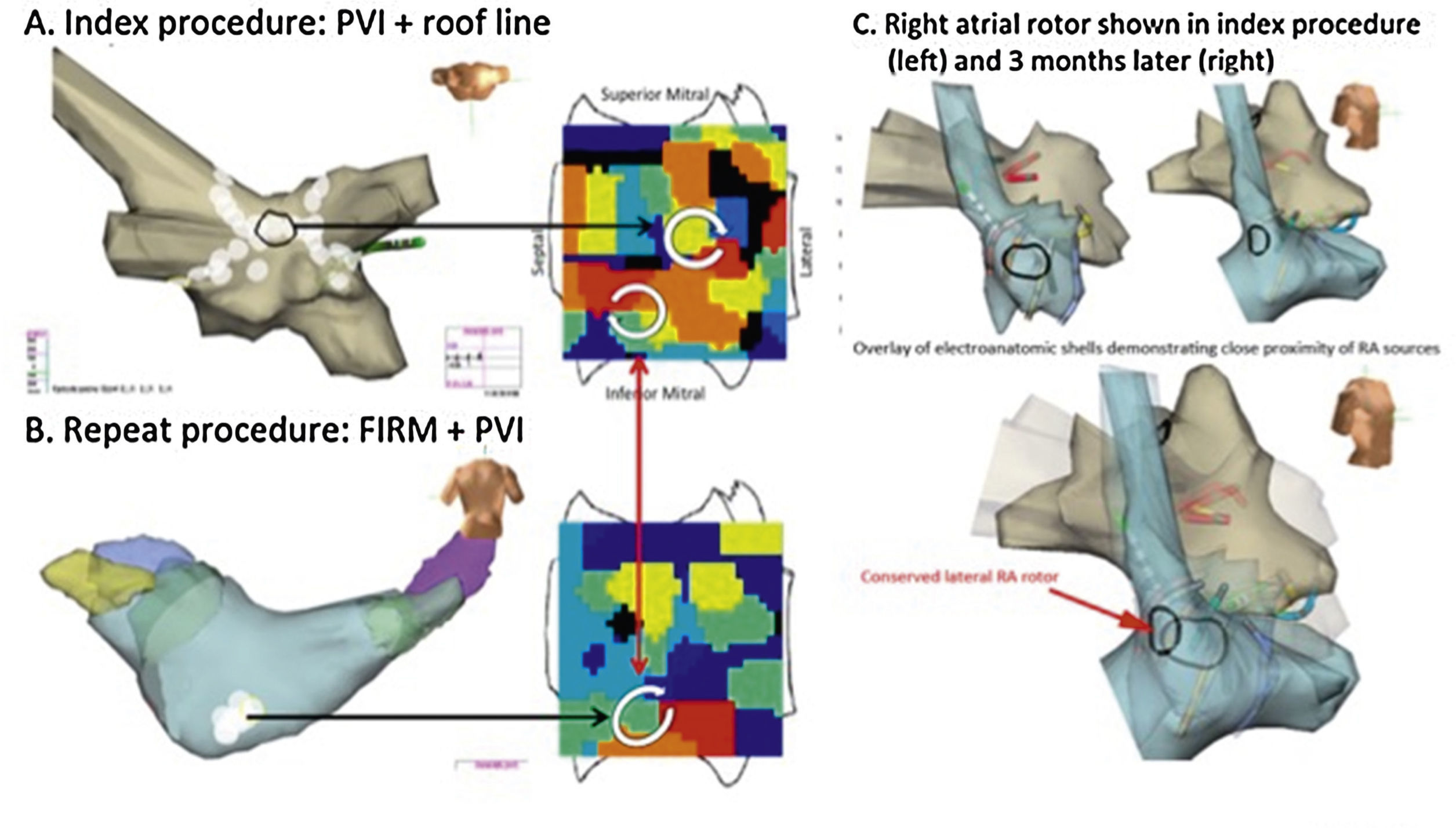
Rotors may drive recurrent AF if not fully eliminated.68 Temporospatial conservation of rotational AF sites in the inferior LA in a man with longstanding persistent AF. (A) At index procedure, anatomic roof line bisected a roof rotor; wide area PVI was also performed. The inferior LA rotor was not prospectively treated by FIRM ablation. (B) During recurrent AF 5 months later, the inferior LA rotor was still present but the previously ablated roof rotor was absent. (C) Right atrial rotor shown in index procedure (left) and 3 months later (right).
Finally, source-guided ablation does not appear to increase complication rates over traditional ablation alone,8,9,54,60 and is likely not pro-arrhythmic.11,12,42,54,55,57,61
In summary, the endpoint of AF source ablation is the elimination of sustained rotational or focal activity that lie in conserved spatial locations. This endpoint is achievable in most patients, and may be superior to historical endpoints of AF termination. Lack of AF termination may reflect many mechanisms including residual AF sources, but clinical results have been promising even in such patients if sources are eliminated.
ConclusionsEvidence continues to mount that human AF is maintained by rotational and focal sources, and that targeting these areas may improve outcomes over PVI alone. We have outlined the scientific rationale for source ablation, and practical strategies for ablation associated with promising outcomes in multicenter studies. Analysis of smaller less promising studies suggests that suboptimal basket placement, resulting in greater difficulty in reading maps and in targeting ablation may explain at least some of these discrepancies. Future improvements in AF source ablation may be facilitated by better computational interpretation of AF maps, comparative studies between potentially complementary mapping approaches, and improvements in basket design. Combined mechanistic and imaging studies may enable better and functional classification of AF that may enable better patient tailoring of AF ablation. Ultimately a better mechanistic and clinical understanding of AF may pave the way for novel drug discovery or regenerative therapies for AF. This is an exciting prospect.
DisclosuresThis work was supported by grants from the National Institutes of Health to Dr Narayan (HL83359, HL103800). Dr. Zaman is supported by a Fulbright foundation award. Dr. Baykaner is supported by a fellowship from the Heart Rhythm Society. Dr. Narayan is co-author of intellectual property owned by the University of California Regents and licensed to Topera Inc., and has held equity in Topera. Dr. Narayan reports consulting fees and honoraria from the American College of Cardiology, Medtronic, St. Jude, Abbott and Uptodate. Dr. Viswanathan reports consulting fees and honoraria from St. Jude Medical, and clinical studies from Medtronic and St. Jude Medical. Dr. Wang reports Honoraria/Consultant from Janssen, St. Jude Medical, Amgen, Medtronic; Fellowship support from Biosense Webster, Boston Scientific, Medtronic, St. Jude Medical; Clinical studies from Medtronic, Siemens, Cardiofocus, ARCA; and Stock options from Vytronus. Drs. Rodrigo and Mr. Kowalewski report no conflicts.