Recently, three novel non-vitamin K antagonist oral anticoagulants received approval for reimbursement in Portugal for patients with non-valvular atrial fibrillation (AF). It is therefore important to evaluate the relative cost-effectiveness of these new oral anticoagulants in Portuguese AF patients.
MethodsA Markov model was used to analyze disease progression over a lifetime horizon. Relative efficacy data for stroke (ischemic and hemorrhagic), bleeding (intracranial, other major bleeding and clinically relevant non-major bleeding), myocardial infarction and treatment discontinuation were obtained by pairwise indirect comparisons between apixaban, dabigatran and rivaroxaban using warfarin as a common comparator. Data on resource use were obtained from the database of diagnosis-related groups and an expert panel. Model outputs included life years gained, quality-adjusted life years (QALYs), direct healthcare costs and incremental cost-effectiveness ratios (ICERs).
ResultsApixaban provided the most life years gained and QALYs. The ICERs of apixaban compared to warfarin and dabigatran were €5529/QALY and €9163/QALY, respectively. Apixaban was dominant over rivaroxaban (greater health gains and lower costs). The results were robust over a wide range of inputs in sensitivity analyses. Apixaban had a 70% probability of being cost-effective (at a threshold of €20000/QALY) compared to all the other therapeutic options.
ConclusionsApixaban is a cost-effective alternative to warfarin and dabigatran and is dominant over rivaroxaban in AF patients from the perspective of the Portuguese national healthcare system. These conclusions are based on indirect comparisons, but despite this limitation, the information is useful for healthcare decision-makers.
Os non-vitamin K antagonist oral anticoagulants (NOAC) foram recentemente comparticipados para a fibrilhação auricular não-valvular (FA), sendo relevante determinar o seu custo-efetividade para a realidade portuguesa.
MétodosFoi especificado um modelo Markov para simular a progressão dos doentes com FA no decurso da sua vida. Os dados de efetividade relativa para os eventos acidente vascular cerebral (isquémico e hemorrágico), hemorragia (intracraniana, outras hemorragias major e hemorragias não-major clinicamente relevantes), enfarte agudo do miocárdio e descontinuação do tratamento foram obtidos por comparações indiretas entre o apixabano, o dabigatrano e o rivaroxabano (comparador comum: varfarina). As fontes dos dados de consumo de recursos de saúde foram a base de dados dos grupos de diagnóstico homogéneo e painel de peritos. Estimou-se os anos de vida ganhos, anos de vida ajustados pela qualidade (QALY), custos diretos e rácios de custo-efetividade incremental (ICER).
ResultadosOs anos de vida ganhos e os QALY foram maiores com apixabano, com um ICER versus varfarina e dabigatrano de 5529 €/QALY e 9163 €/QALY, respetivamente. O apixabano foi dominante versus o rivaroxabano (maiores ganhos em saúde e menores custos). Estes resultados foram robustos nas análises de sensibilidade realizadas, tendo o apixabano uma probabilidade de 70% de ser custo-efetivo (threshold: 20000 €/QALY) versus o conjunto das restantes opções terapêuticas.
ConclusõesA utilização de apixabano em doentes com FA na prática clínica portuguesa é custo-efetiva versus varfarina e dabigatrano e dominante versus rivaroxabano na perspetiva do SNS. Estas conclusões baseiam-se em comparações indiretas. Apesar desta limitação, esta informação é relevante para os diferentes decisores em saúde.
confidence interval
clinically relevant non-major bleeding
center time in therapeutic range
cardiovascular
diagnosis-related groups
gastrointestinal
hazard ratio
intracranial hemorrhage
incremental cost-effectiveness ratio
Portuguese National Institute of Statistics
international normalized ratio
indirect treatment comparison
myocardial infarction
modified Rankin scale
National Health Service
non-vitamin K antagonist oral anticoagulants
odds ratio
quality-adjusted life years
relative risk
time in therapeutic range
vitamin K antagonists
Atrial fibrillation (AF) is the most prevalent arrhythmia in clinical practice. It is estimated that 2.5% of the Portuguese population over the age of 40 and more than 10% of those aged over 80 have AF.1 Since it can be asymptomatic and remain undiagnosed until a complication occurs (ischemic stroke or systemic embolism),2,3 screening is currently recommended for those aged over 65 years. Diagnosis of the condition is essential in order to stratify thromboembolic risk and to decide whether to prescribe prophylactic medication. Oral anticoagulation with vitamin K antagonists (VKAs) is the mainstay of pharmacological intervention for this purpose and reduces stroke risk by over 50% in patients with AF.4 However, despite their demonstrated efficacy in clinical trials, the use of these drugs has consistently been reported as suboptimal.5
Recently, new pharmacological options have been developed with the same therapeutic goals, notably the non-vitamin K antagonist oral anticoagulants known as the new oral anticoagulants (NOACs). They are considered at least as effective as VKAs, with lower risk of intracranial hemorrhage7 and with no need for laboratory monitoring of international normalized ratio (INR). Three of these NOACs have been approved to date for reimbursement under the National Health Service (NHS) for AF patients in Portugal: apixaban, dabigatran and rivaroxaban. These three drugs have different mechanisms of action, pharmacokinetics and dosage regimens, and thus offer different therapeutic options for individual patients according to renal dysfunction, age, bleeding risk, history of coronary artery or peripheral arterial disease, and stroke risk.
Although these drugs are more expensive than VKAs, studies on dabigatran and rivaroxaban compared to warfarin for AF patients in Portugal indicate that they are cost-effective in clinical practice.8,9 Since August 1, 2014, these NOACs have been reimbursed by the NHS for the prevention of thromboembolic events in patients with non-valvular AF. Against this background, it is important for decision-makers to be aware of the health gains and associated costs of the different NOACs. The aim of this study was thus to estimate the cost-effectiveness of NOACs, particularly apixaban (the most recent to have obtained market authorization) compared to warfarin, dabigatran and rivaroxaban.
MethodsModel structureA Markov model of cost-effectiveness and cost-utility was used, with a six-week cycle length, the minimum period in which changes in health (or disease) state would be expected, following a cohort of 1000 patients over a lifetime horizon. The model, details of which were recently published by Lip et al.10, was programmed in Excel using Visual Basic for Applications (Figure 1).
Markov model decision tree. AC: anticoagulants; ASA: aspirin; CRNM: clinically relevant non-major; HS: hemorrhagic stroke; ICH: intracranial hemorrhage; IS: ischemic stroke; NVAF: non-valvular atrial fibrillation; NVAF subsequent ASA: NVAF patients on second-line aspirin. Reproduced from Lip et al.10
In the model, the natural history of the disease is represented by 11 mutually exclusive health states: non-valvular AF; mild, moderate or severe non-fatal ischemic stroke; mild, moderate or severe non-fatal hemorrhagic stroke; systemic embolism; myocardial infarction (MI); non-valvular AF with discontinued first-line anticoagulation; and death. After six weeks the patient can enter, remain in, or transition to another state according to the corresponding transition probability, defined as the likelihood of an event occurring within that period.
The risk of ischemic stroke is calculated according to the patient's CHADS2 score11 (the method for estimating thromboembolic risk in use at the time of the clinical trials of the drugs under analysis) and the level of anticoagulation for patients treated with warfarin as determined by time in therapeutic range (TTR) of the international normalized ratio (INR). The likelihood of stroke, MI, intracranial bleeding and other major and non-major bleeding increases with age. The model also considers the long-term impact of MI and systemic embolism on mortality, reflected in higher hazard ratios (HR). For patients in the state of non-valvular AF who discontinue first-line anticoagulation, the model structure remains the same but the transition probabilities differ.
Severity of stroke (ischemic or hemorrhagic) is classified according to the modified Rankin scale (mRS)12: mild, 0–2; moderate, 3–4; severe, 5; and fatal, 6. All patients with fatal stroke transition to the state of death in the following cycle, while non-fatal stroke is modeled as a tunnel state from which patients can only transition to recurrent stroke or death. Patients can only experience one recurrent stroke in the model, from which the transition is to stroke of the same or greater severity. The model does not allow recurrent MI or systemic embolism, patients either remaining in the same health state or transitioning to death.
At the end of each cycle health care costs, quality-adjusted life years (QALYs) and life years gained are calculated. Levels of health-related quality of life (utilities), clinical outcomes and mortality rates vary according to stroke severity. In accordance with the Portuguese Ministry of Health's guidelines for economic evaluation studies of drugs,13 published by Infarmed, costs and utilities are discounted at an annual rate of 5%.
PopulationIn the model's base-case scenario, the characteristics of the population are those of patients enrolled in trials of apixaban, specifically ARISTOTLE,14 in terms of median age (70 years), gender (64.7% male), and distribution of CHADS2 scores (1–2: 69%; 3–4: 27%; and 5–6: 4%).
ComparatorsThe results of treatment with apixaban 2.5–5 mg twice daily are compared with (1) dabigatran 150 mg twice daily in patients aged ≤80 years and 110 mg twice daily in patients aged >80 years with high bleeding risk and those treated with verapamil (the dabigatran group) and (2) rivaroxaban 15–20 mg once daily.
Relative effectiveness of the new oral anticoagulants: indirect comparisonsEconomic evaluations of new health technologies such as drugs analyze their effectiveness and the associated costs compared to existing options. Assessment of the relative effectiveness of the NOACs is thus one of the central aims of this study. There have to date been no head-to-head studies between the NOACs, so their effectiveness in AF must be estimated by indirect analysis using a common comparator, in this case warfarin.
It is therefore essential to assess the reliability of the estimates of effectiveness used in the model. To this end we carried out a systematic review of the literature to identify indirect comparisons between NOACs that provide data on their effectiveness in AF, searching the MEDLINE and Cochrane Library databases in September 2014 using the search terms meta-analysis, indirect comparison, bayesian, network, apixaban, dabigatran, rivaroxaban and atrial fibrillation. Ten studies were identified, six frequentist10,15–19 and four Bayesian (network meta-analyses).6,20–22
Table 1 summarized the characteristics of each of these studies. As can be seen in Figure 2, the estimates for the various outcomes in these publications are consistent and are similar to those used in the base-case scenario in the economic model.10 Given the aim of the present study, Lip et al.10 (frequentist indirect comparison using the method of Bucher et al.23) and Mitchell et al.20 (Bayesian network meta-analysis) probably give the best estimates of the relative effectiveness of the three NOACs in AF, since they use only data from phase III clinical trials and establish associations using HRs, which takes the time factor into account and respects the primary statistical analysis of each trial. Supplementary Figure 3 (Annex) shows the evidence network used for these two indirect comparisons.
Characteristics of published indirect comparisons between new oral anticoagulants in atrial fibrillation.
| Study | Association measure | Clinical trials included |
|---|---|---|
| Frequentist indirect comparisons | ||
| Lip et al.10 | HR | RE-LY, ROCKET AF, ARISTOTLE |
| Testa et al.17 | OR | RE-LY, ROCKET AF, ARISTOTLE |
| Harenberg et al.16 | OR | RE-LY, ROCKET AF, ARISTOTLE |
| Baker et al.15 | RR | RE-LY, ROCKET AF, ARISTOTLE, PETRO |
| Lip et al.18 | HR | RE-LY, ROCKET AF, ARISTOTLE |
| Bayesian network meta-analyses | ||
| Mitchell et al.20 | HR | RE-LY, ROCKET AF, ARISTOTLE |
| Assiri et al.22 | RR | RE-LY, ROCKET AF, ARISTOTLE, 18 other RCTs |
| Dogliotti et al.6 | OR | RE-LY, ROCKET AF, ARISTOTLE, AVERROES, ACTIVE-W, ACTIVE-A 11 comparisons vs. placebo |
| Cameron et al.21 | OR | RE-LY, ROCKET AF, ARISTOTLE, ARISTOTLE J, ENGAGE AF AVERROES, ACTIVE-W, ACTIVE-A Comparisons vs. placebo |
HR: hazard ratio; OR: odds ratio; RR: relative risk.
Estimates of effectiveness of apixaban compared to other new oral anticoagulants in published indirect comparisons. A: apixaban; B: Bayesian network meta-analysis; D: dabigatran; ITC: indirect treatment comparison; R: rivaroxaban. *The RE-LY study only presents results for minor bleeding, which were used as a proxy for clinically relevant non-major bleeding.
The event rates in the base-case scenario are derived from the HRs reported by Lip et al.10 (Table 2). The distribution of stroke events by severity is presented in Supplementary Table 3.
Hazard ratios (95% confidence interval): apixaban vs. warfarin and other new oral anticoagulants.
| Apixaban | Warfarin | Dabigatran 110 mg | Dabigatran 150 mg | Rivaroxaban | |
|---|---|---|---|---|---|
| Ischemic stroke | 1.00 | 1.09 (0.89–1.35) | 1.20 (0.88–1.64) | 0.82 (0.60–1.14) | 0.98 (0.72–1.33) |
| ICHa | 1.00 | 2.38 (1.72–3.33) | 0.73 (0.43–1.26) | 1.02 (0.62–1.68) | 1.73 (1.08–2.77) |
| Systemic embolism | 1.00 | 1.00 (0.90–1.10)b | 1.00 (0.90–1.10)b | 1.00 (0.90–1.10)b | 1.00 (0.90–1.10)b |
| Other major bleeding | 1.00 | 1.27 (1.08–1.47) | 1.21 (0.97–1.50) | 1.37 (1.10–1.71) | 1.44 (1.15–1.79) |
| CRNMB | 1.00 | 1.43 (1.24–1.66) | 1.16 (0.99–1.35) | 1.30 (1.11–1.53) | 1.49 (1.26–1.76) |
| MI | 1.00 | 1.14 (0.86–1.52) | 1.47 (0.96–2.27) | 1.46 (0.95–2.24) | 0.94 (0.64–1.38) |
| Other CV hospitalizations | 1.00 | 1.00 (0.90–1.10)c | 1.00 (0.90–1.10)c | 1.00 (0.90–1.10)c | 1.00 (0.90–1.10)c |
CRNMB: clinically relevant non-major bleeding; CV: cardiovascular; ICH: intracranial hemorrhage; MI: myocardial infarction.
Intracranial hemorrhage includes hemorrhagic stroke and other types of intracranial hemorrhage. The proportion of hemorrhagic stroke among intracranial hemorrhage was 77%, 64%, 64%, 41% and 57% for apixaban, warfarin, dabigatran 110 mg, dabigatran 150 mg and rivaroxaban, respectively, according to published studies (secondary analyses of the ARISTOTLE, RE-LY and ROCKET AF trials).
As stated above, the risk of ischemic stroke and bleeding events associated with warfarin depends on the level of anticoagulation as determined by INR (Supplementary Table 4). The model classifies patients in four categories according to various cutoffs for median center time in therapeutic range (cTTR) based on the results of centers participating in the ARISTOTLE trial. The distribution is uniform, with 25% of patients in each category.
To parametrize the model to reflect the situation in Portugal, we used data from a convenience sample of patients attending the anticoagulation clinics at two hospitals in the Lisbon region, Centro Hospitalar Lisboa Central and Hospital Fernando da Fonseca. These data are from 2011 and 2012 and are on patients who underwent at least 10 INR measurements, with a total of 39630 measurements in 2850 patients, and were used to calculate patient median TTR. The median cTTR could not be estimated since the data are limited to two hospitals, but the above median TTR was considered a reasonable approximation to the cTTR defined in the model. The robustness of the results obtained from this hospital sample was checked by comparing them with a sample of 4470 outpatient INR measurements in 233 patients; no statistically significant differences were found.
On the basis of these data, anticoagulation levels in the Portuguese population are lower than considered in the model, since only 44.5% of Portuguese patients have TTR ≥52.38%, as opposed to 75% with ≥52.38% in the trials (Supplementary Table 5).
Treatment discontinuation rates (%/year) due to non-vascular causes were obtained from a secondary analysis of the ARISTOTLE trial results (13.2% with apixaban and 14.4% with warfarin), assuming constant rates over time. Supplementary Table 6 shows HRs for treatment discontinuation for reasons other than vascular events. Second-line treatment was assumed to be aspirin. Absolute risks associated with events per 100 patient/years are summarized in Supplementary Table 7.
CostsThe study adopts the perspective of the NHS and therefore does not analyze indirect costs. Three main types of costs are identified in the model: costs arising from vascular events, costs of anticoagulant therapy, and costs of monitoring and/or routine consultations. Costing is based on (1) Order in Council 20/201424 for unit costs of consultations, diagnostic exams and diagnosis-related groups (DRGs); (2) analysis of the database of NHS hospitalizations (DRGs) in 201325; (3) the Portuguese Ministry of Health's drug database (Infomed) for prices of medications, consulted on January 2, 201526; and (4) estimates outpatient care resource use by a geographically representative expert panel of various specialists. For the health states of non-fatal ischemic or hemorrhagic stroke, MI and systemic embolism, costs were divided into acute and long-term maintenance, the acute phase including the first two weeks of hospital stay and the first three months of rehabilitation. The model assumes that the maintenance stage will continue until death and according to the expert panel, includes costs of consultations, emergencies and transport, diagnostic exams, medication and technical assistance. It was not possible to estimate the costs of stroke according to severity (mild, moderate or severe), since there are no data on costs according to the mRS in Portugal. For the other health states only the costs of hospitalization (acute phase) were included.
Overall costs per event, treatment costs and costs of monitoring and routine care are shown in Table 3.
Costs arising from vascular events, anticoagulant therapy and monitoring and routine consultations.
| Events | Costs (€) | |
|---|---|---|
| Acute (per episode) | Long-term (per month) | |
| Non-fatal ischemic stroke (weighted mean) | 8653.26 | 44.57 |
| Fatal ischemic stroke | 6381.20 | – |
| Non-fatal hemorrhagic stroke (weighted mean) | 13779.62 | 41.07 |
| Fatal hemorrhagic stroke | 10419.64 | |
| Other intracranial hemorrhage | 7932.21 | – |
| GI bleeding | 8798.64 | – |
| Non-intracranial and non-GI bleeding | 2090.04 | – |
| CRNMB | 2514.98 | 42.32 |
| Systemic embolism | 3937.93 | – |
| MI | 4560.10 | 53.61 |
| Other CV hospitalizations | 2081.64 | – |
| Medication | Mean daily costa | Monitoring and routine care | |
|---|---|---|---|
| Monthly frequency | Costc | ||
| Warfarin | 0.08 | 0.92b | 31.00 |
| Apixaban | 2.41 | 0.30d | 31.00 |
| Dabigatran 110 mg | 2.36 | 0.30d | 31.00 |
| Dabigatran 150 mg | 2.46 | 0.30d | 31.00 |
| Rivaroxaban | 2.47 | 0.30d | 31.00 |
Source: bdatabases of Centro Hospitalar Lisboa Central and Hospital Fernando da Fonseca; cOrder in Council 20/201424; dexpert panel.
The probabilities of death associated with vascular events in the model are those observed in the trials of the NOACs, with the exception of the fatality rate in MI, which was obtained from Scarborough et al.27 The model assumes that these probabilities are independent of treatment. For the period corresponding to the duration of the ARISTOTLE trial, mortality from non-vascular causes is assumed to be the same for all three NOACs, and the figures in the ARISTOTLE trial (3.08% for apixaban and 3.34% for warfarin) was used in the model. Mortality after the period analyzed in the clinical trials was estimated on the basis of Portuguese life tables,28 multiplied by the HRs associated with the population with AF estimated by Friberg et al. to take into account the increased risk of this population.29 Specifically, the parameters of a Gompertz survival function were calculated by age-group (<75 years and ≥75 years) and by gender. The model includes adjustments to mortality risk to account for the increased mortality associated with AF and different degrees of stroke severity (Supplementary Table 9).
Health-related quality of life weights or utilitiesThe mean values for utilities and disutilities associated with different health states were taken to be the same as those estimated for the UK population by Sullivan et al.30 There are also disutilities associated with warfarin therapy (unlike the NOACs) and with other vascular events. The model assumes that these disutilities are cumulative. Table 4 summarizes the utilities and disutilities used in the model.
Mean utilities and disutilities for the population in the model.
| Utility considered in the model for each health statea | |
| AF (baseline utility) | 0.7270 |
| Stroke (ischemic and hemorrhagic) | |
| Mild | 0.6151 |
| Moderate | 0.5646 |
| Severe | 0.5142 |
| Systemic embolism | 0.6265 |
| MI | 0.6098 |
| Disutilities associated with therapy and with other vascular events (duration) | |
| Anticoagulants | |
| Warfarinb | 0.0130c |
| NOACs | 0.0000c |
| Events | |
| Other ICH (excluding hemorrhagic stroke) | 0.1511 (6 weeks) |
| Other major bleeding (excluding ICH) | 0.1511 (14 days) |
| CRNMB | 0.0582 (2 days) |
| Other CV hospitalizations | 0.1276 (6 days) |
One-way sensitivity analyses were performed to assess the robustness of the results in terms of the following parameters: (1) use of the HRs estimated by Mitchell et al. (Bayesian network meta-analysis) instead of those estimated by Lip et al.10; (2) anticoagulation levels as reported in the clinical trials, instead of those obtained in Portuguese patients; (3) duration of the acute phase of hospitalization taken to be six rather than two weeks; (4) different costs of stroke depending on severity, with weighting calculated on the basis of UK figures, instead of a uniform cost for stroke of any severity; (5) the same distribution of stroke of similar severity for all NOACs (based on the distribution in the case of apixaban); (6) the same treatment discontinuation rates for non-vascular causes for all comparators as for apixaban (13.2%/year) from the beginning of treatment, instead of the rates reported in the clinical trials; (7) mortality rates after the period covered by the trials taken to be the same as for the general population, thus underestimating mortality; (8) use of different utilities associated with each health state, as estimated in a previous study by Sullivan et al.,46 and used in other studies of the cost-effectiveness of NOACs32–34; and (9) a discount rate for costs and utilities of 0% or 3% instead of 5%.
A probabilistic sensitivity analysis using 2000 Monte Carlo simulations incorporating second-order uncertainty was also performed.35 The results are presented as the probability of apixaban being cost-effective compared to the other therapeutic options based on a threshold of €20000/QALY, the limit usually taken to be acceptable for funding new health technologies in Portugal.
ResultsEvent rates and costsTable 5 shows the number of vascular events associated with each anticoagulant in a cohort of 100000 patients according to the rates derived from the model. The number of vascular events and event-related deaths was lower with apixaban except for hemorrhagic stroke. The difference was greatest for ischemic stroke, other major bleeding, clinically relevant non-major bleeding and event-related deaths.
Event rates for each therapeutic option per 100000 patients.
| Number of events (total population) | Apixaban | Warfarin | Dabigatran | Rivaroxaban |
|---|---|---|---|---|
| Ischemic stroke | ||||
| Non-fatal | 19799 | 20703 | 20066 | 19649 |
| Fatal | 2932 | 2857 | 3392 | 3283 |
| Total | 22731 | 23560 | 23458 | 22931 |
| Hemorrhagic stroke | ||||
| Non-fatal | 1602 | 2040 | 996 | 1879 |
| Fatal | 1007 | 2171 | 702 | 938 |
| Total | 2609 | 4212 | 1698 | 2818 |
| Systemic embolism | ||||
| Non-fatal | 2138 | 2175 | 2403 | 2263 |
| Fatal | 221 | 225 | 249 | 234 |
| Total | 2359 | 2400 | 2652 | 2497 |
| Other IC hemorrhage | ||||
| Non-fatal | 1063 | 2255 | 1521 | 1901 |
| Fatal | 159 | 337 | 227 | 284 |
| Total | 1221 | 2591 | 1748 | 2185 |
| Other major bleeding | ||||
| Non-fatal GI bleeding | 5055 | 5713 | 7501 | 8338 |
| Non-fatal non-intracranial and non-GI bleeding | 8137 | 10123 | 8984 | 10802 |
| Fatal | 269 | 326 | 336 | 391 |
| Total | 13461 | 16159 | 16822 | 19530 |
| CRNMB | 25248 | 30700 | 29914 | 33367 |
| MI | ||||
| Non-fatal | 7179 | 7345 | 8366 | 7182 |
| Fatal | 1043 | 1067 | 1214 | 1044 |
| Total | 8222 | 8412 | 9579 | 8226 |
| Other CV hospitalizations | 116048 | 112390 | 117558 | 116738 |
| Other reasons for treatment discontinuation | 63406 | 62408 | 72720 | 66616 |
| Deaths | ||||
| Event-related (acute) | 5940 | 7332 | 6364 | 6480 |
| Event-related (due to stroke, MI, or systemic embolism) | 30524 | 32066 | 31694 | 30779 |
| Other | 63536 | 60602 | 61942 | 62741 |
| Total | 100000 | 100000 | 100000 | 100000 |
CRNMB: clinically relevant non-major bleeding; CV: cardiovascular; MI: myocardial infarction; IC: intracranial; GI: gastrointestinal.
Table 6 and Figure 3 present the breakdown of costs associated with the different therapeutic options over a lifetime horizon. Warfarin has the lowest mean cost per patient and rivaroxaban the highest. The total mean cost of apixaban is between these two, with the lowest clinical costs (due to its low vascular event rate) and lowest costs of monitoring and routine care. Although the daily cost of apixaban is lower than dabigatran and rivaroxaban, lifetime costs are greater because the duration of treatment tends to be longer due to its lower discontinuation rate.
Total mean cost per patient for each therapeutic option over a lifetime horizon.
| Costs (in €) | Warfarin | Apixaban | Dabigatran | Rivaroxaban |
|---|---|---|---|---|
| Clinical events | 5467.29 | 4989.03 | 5244.03 | 5386.30 |
| Therapy | 214.42 | 3754.35 | 3015.69 | 3463.96 |
| Monitoring and routine care | 3252.29 | 1254.77 | 1311.27 | 1278.31 |
| Total | 8934.16 | 9998.14 | 9570.99 | 10128.56 |
Table 7 and Figure 4 show the results of the cost-effectiveness analysis of apixaban compared to the other therapeutic options. As suggested in the literature36,37 for multiple comparisons, the results are presented as a graph in which the x-axis represents the differences in QALYs and the y-axis the differences in cost between the comparators and the reference therapy (in this case warfarin). The red line linking the points on the graph represents the efficient frontier. The frontier consist of three segments: its slope corresponds to €4367/QALY when it joins the points representing warfarin and dabigatran, €9163/QALY when it joins the points representing dabigatran and apixaban, and is vertical above apixaban because no therapy is more effective. Rivaroxaban is dominated because it is to the left of the cost-effectiveness frontier, presenting greater costs and fewer QALYs than other therapies on the frontier. Rivaroxaban is also dominated by apixaban when analyzed in isolation.
Cost-effectiveness analysis of apixaban compared to the other therapeutic options in the base-case scenario.
| Apixaban compared to | |||
|---|---|---|---|
| Warfarin | Dabigatran | Rivaroxaban | |
| Incremental costs | €1063.98 | €427.15 | –€130.42 |
| Life years gained | 0.19 | 0.05 | 0.04 |
| Incremental QALYs | 0.19 | 0.05 | 0.03 |
| ICER | |||
| Cost per life year gained | €5708.44 | €7926.91 | Dominant |
| Cost per QALY gained | €5529.05 | €9162.77 | Dominant |
ICER: incremental cost-effectiveness ratio; QALYs: quality-adjusted life years.
Effectiveness (measured in quality-adjusted life years) and incremental costs of the new oral anticoagulants (NOACs) relative to warfarin (represented by the coordinates 0,0). The red line represents the efficient frontier; the slope of each segment corresponds to the incremental cost-effectiveness ratio between the points defining that segment. NOACs with fewer incremental QALYs are to the left and those with greater incremental costs are higher. The incremental cost of apixaban is €1064 compared to warfarin but it is more cost-effective than the other therapeutic options. Points to the left of the line are dominated by therapies that are more effective than at the frontier, and so rivaroxaban, with fewer QALYs and greater costs, is dominated by apixaban. ICER: incremental cost-effectiveness ratio; QALYs: quality-adjusted life years.
The results of the one-way and probabilistic sensitivity analyses confirm the robustness of the study's findings. In the one-way analysis of the nine parameters specified in the Methods section, which reflect a range of alternate scenarios, apixaban is always dominant compared to rivaroxaban. Compared to the other therapeutic options, apixaban presents ICERs well below €20000/QALY, ranging between €4909 and €6741/QALY compared to warfarin and between €5162 and €12016/QALY compared to dabigatran. If it is assumed that discontinuation rates for non-vascular causes remain the same from the beginning of treatment, the costs of apixaban are less than either rivaroxaban or dabigatran. In this case, apixaban is dominant compared to rivaroxaban and, for a threshold of €20000/QALY, is cost-effective compared to warfarin and dabigatran. The results of the sensitivity analyses are summarized in Supplementary Table 14.
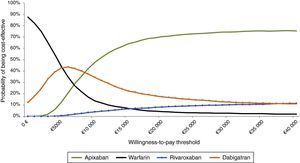
In the probabilistic sensitivity analysis, the probability of apixaban being cost-effective for a threshold of €20000/QALY is 96%, 87% and 95% compared to warfarin, dabigatran and rivaroxaban, respectively. If all the comparators are considered together (Figure 5), apixaban is the best alternative from a threshold of €8000/QALY. In this scenario, for a willingness to pay of €20000/QALY, the probability of apixaban being cost-effective is 70%.
Cost-effectiveness acceptability curves showing the percentage of simulations for each willingness-to-pay value that are cost-effective for each treatment, enabling simultaneous comparison between all the therapeutic options. Apixaban is the best alternative from €8000/QALY. For a willingness to pay of €20000/QALY, the probability of apixaban being cost-effective compared to all the other alternatives is 70%.
AF is the most common arrhythmia1 and has a considerable social impact due to associated mortality and morbidity. In Portugal, it has been estimated that 3.8% of all deaths in 2010 could be attributed to AF, and that in terms of overall burden of disease and cost of illness, it was responsible for around 23000 disability-adjusted life years and total costs of around €140 million in 2010, about 0.08% of gross national product.38 These figures are expected to rise as the incidence of AF increases with aging populations and greater prevalence of chronic heart disease, among other factors.39 More widespread use of ambulatory electrocardiographic monitoring has improved diagnosis and will also help ensure that significant health gains will continue to be made in AF patients in the future.
Antithrombotic therapy, particularly anticoagulation, significantly reduces the risk of AF-related thromboembolic events, especially stroke.4 There were few therapeutic options for this purpose for several decades, when warfarin was the reference treatment, but the development of NOACs has changed the picture. Since the NHS began reimbursing these drugs the number of patients using them has increased significantly, and it is likely that NHS spending on outpatient anticoagulation therapy (currently 4.5%, corresponding to more than €50 million in 2014) will rise further.40 In the light of this situation, it is important for health decision-makers to have access to estimates of the cost-effectiveness of these NOACs for stroke prevention in AF.
Several cost-effectiveness studies have been published in which a specific NOAC was compared with warfarin. Without exception these studies, carried out in both Europe and the USA, have shown that the NOACs are cost-effective compared to warfarin.41 However, the results of these studies cannot be used for naive indirect cost-effectiveness comparisons, and they certainly do not reflect the situation in Portugal. We therefore performed an economic evaluation based on a previously published model10 that compared the three NOACs to each other, which was adapted for the clinical setting.
The results of the present study show that apixaban is cost-effective compared to warfarin and dabigatran (ICERs of €5529/QALY and €9163/QALY, respectively) and dominant compared to rivaroxaban. The probability of apixaban being cost-effective compared to all the other therapeutic options is 70% for a threshold of €20000/QALY. These results are in agreement with those of studies in other European contexts, including Belgium,42 the Netherlands,32 the UK10,43 and France,44 in which apixaban was also cost-effective compared to warfarin and cost-effective or dominant compared to dabigatran and rivaroxaban. The fact that apixaban is the most cost-effective NOAC in these studies may be due to its greater effectiveness, which can be attributed to the lower rate of vascular events associated with its use, particularly ischemic stroke,10,44,45 major bleeding20 and event-related deaths.10,20 A logical consequence is that apixaban presents a lower event-related discontinuation rate and that patients remain under treatment for longer (and thereby benefit in terms of thromboembolic prevention). This lower discontinuation rate explains the higher total lifetime costs of apixaban therapy compared to the other NOACs.
However, other studies have recently been published, in Norway33 and the UK,32,34 in which the results are different, with dabigatran being considered cost-effective compared to apixaban (both being superior to rivaroxaban). In these studies, incremental QALYs were 0.2%–1.3% higher with dabigatran than with apixaban, even though the numbers of vascular events used in the analysis were taken from the same clinical trials as those used in the present study.
Various methodological differences may account for these conflicting results: (1) differences in modeling; (2) use of different non-event-related discontinuation rates; (3) modeling of mortality after the trial period; (4) use of different values for the utilities associated with each health state (the present study uses estimates based on Sullivan et al. in 2011,30 while the other studies were based on the values reported by the same group in 200646); (5) different discount rates.
All of these differences except the first were subjected to one-way sensitivity analysis in the present study that confirmed the robustness of the main results. Therefore, the differences between the studies cannot be explained by these parameters. They may thus be due to differences in modeling, including the ways in which the states of the Markov model are specified, different cycle lengths, the use of a single level of severity for stroke, and differences in cost estimates (which are influenced by the resources and characteristics of health care systems and the prices of drugs in each country). A quantitative analysis of these questions is beyond the scope of this study.
Some studies have suggested that the cost-effectiveness of the NOACs depends on the level of anticoagulation control, in that they will tend to be more cost-effective when anticoagulation control is poor. In particular, it has been suggested that dabigatran is less cost-effective in well-controlled patients.47,48 However, the results of sensitivity analysis for this parameter showed no significant differences.
This study has certain limitations in terms of the data used, particularly for the number of events, since these were taken from clinical trials with short follow-up periods (2–3 years), which may not reflect the actual effectiveness of each drug. Furthermore, in the absence of head-to-head comparisons between the NOACs, cost-effectiveness was estimated indirectly, using warfarin as a common comparator, and so it was not possible to control for differences in baseline patient characteristics, trial design, anticoagulation level or risk profile determined by CHADS2 score (although the results on the cost-effectiveness of apixaban are similar in the subpopulation with higher CHADS2 scores).49 According to the literature review carried out by the authors of the present study, the estimates of effectiveness used in this study are consistent with those in published indirect comparisons and the results did not change when other estimates obtained by Bayesian methods were used.20
ConclusionIn this cost-effectiveness analysis based on indirect comparisons, apixaban was cost-effective compared to warfarin and dabigatran and dominant over rivaroxaban in patients with non-valvular AF. These conclusions were robust in all the sensitivity analyses performed. This information is useful for healthcare decision-makers when selecting the best option for the individual patient.
Ethical disclosuresProtection of people and animalsThe authors declare that no experiments were performed on humans or animals for this study.
Data confidentialityThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThe study was funded by Bristol-Myers Squibb Farmacêutica Portuguesa, SA and Laboratórios Pfizer Lda. Funding was independent of the study outcome. Mónica Inês is an employee of Laboratórios Pfizer Lda.
Conflict of interestThe authors have no other conflicts of interest to declare.
Please cite this article as: Costa J, Fiorentino F, Caldeira D, Inês M, Lopes Pereira C, Pinheiro L, et al. Custo-efetividade dos novos anticoagulantes orais na fibrilhação auricular em Portugal. Rev Port Cardiol. 2015;34:723–737.
















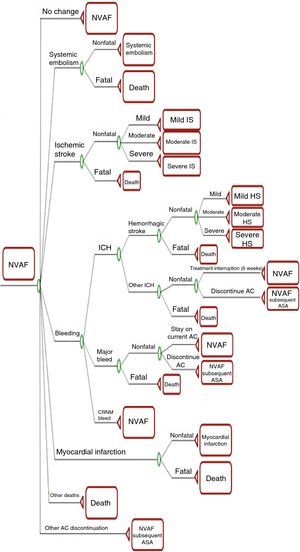 ICH: intracranial hemorrhage; IS: ischemic stroke; NVAF: non-valvular atrial fibrillation; NVAF subsequent ASA: NVAF patients on second-line aspirin. Reproduced from Lip et al.10'/>
ICH: intracranial hemorrhage; IS: ischemic stroke; NVAF: non-valvular atrial fibrillation; NVAF subsequent ASA: NVAF patients on second-line aspirin. Reproduced from Lip et al.10'/> ITC: indirect treatment comparison; R: rivaroxaban. *The RE-LY study only presents results for minor bleeding, which were used as a proxy for clinically relevant non-major bleeding.'/>
ITC: indirect treatment comparison; R: rivaroxaban. *The RE-LY study only presents results for minor bleeding, which were used as a proxy for clinically relevant non-major bleeding.'/>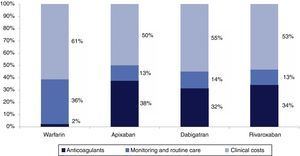
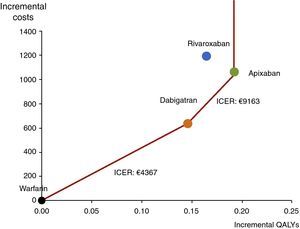 NOACs) relative to warfarin (represented by the coordinates 0,0). The red line represents the efficient frontier; the slope of each segment corresponds to the incremental cost-effectiveness ratio between the points defining that segment.
NOACs) relative to warfarin (represented by the coordinates 0,0). The red line represents the efficient frontier; the slope of each segment corresponds to the incremental cost-effectiveness ratio between the points defining that segment.