Individuals with glomerular filtration rate (GFR) ≥60 ml/min/1.73 m2 estimated by the Cockcroft-Gault formula (CG) who undergo percutaneous coronary intervention (PCI) frequently develop contrast-induced nephropathy (CIN). This study aimed to assess whether individuals with significant renal impairment assessed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, but not by CG, more often develop CIN following PCI than those without renal impairment by either formula.
MethodsIn this cross-sectional study analyzing patients with baseline CG GFR ≥60 ml/min/1.73 m2 before PCI, subjects were divided into two groups according to CIN occurrence. Baseline CKD-EPI GFR was calculated for all patients.
ResultsWe analyzed 140 patients. Baseline GFR was 87.5±21.3 and 77.1±15.0 ml/min/1.73 m2 for CG and CKD-EPI, respectively. CIN occurred in 84.6% of individuals with baseline CKD-EPI GFR <60 ml/min/1.73 m2 vs. 51.1% of those without. Males and those with higher body mass index were more likely to present baseline CKD-EPI GFR <60 ml/min/1.73 m2 (p=0.021). Non-ionic contrast agent use and baseline CKD-EPI GFR ≥60 ml/min/1.73 m2 were protective factors against CIN. Greater amounts of contrast agent and acute coronary syndrome were associated with higher CIN risk. In subjects with serum creatinine <1.0 mg/dl, GFR was more likely to be overestimated by CG, but not by CKD-EPI (sensitivity 100.0%; specificity 52.0%).
ConclusionIn patients undergoing PCI without renal dysfunction by CG, a finding of CKD-EPI GFR <60 ml/min/1.73 m2 was associated with a higher probability of CIN, especially among men and those with higher body mass index.
Nefropatia induzida por contraste (NIC) após intervenção coronária percutânea (ICP) em pacientes com taxa de filtração glomerular (TFG) ≥60 mL/min, estimada pela equação de Cockcroft-Gault (C-G), não é infrequente. O objetivo desse estudo foi avaliar a capacidade da equação CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) em predizer NIC em indivíduos sem disfunção renal significativa basal pela fórmula C-G.
MétodosIncluídos pacientes submetidos a ICP entre 2008-2013, com TFG basal ≥60 mL/min pela equação de C-G. Estes indivíduos foram divididos em dois grupos, conforme ocorrência ou não de NIC. Para todos os casos, foi calculada a TFG basal conforme a equação do CKD-EPI.
ResultadosA amostra consistiu de 140 pacientes. A TFG C-G basal foi de 87,5±21,3 mL/min e de 77,1±15,0 mL/min/1,73 m2 para CKD-EPI. NIC ocorreu em 84,6% dos pacientes com TFG CKD-EPI basal <60 mL/min/1,73 m2, contra 51,1% daqueles com TFG CKD-EPI basal ≥60 mL/min/1,73 m2 (p=0,021). Indivíduos masculinos ou com peso corporal elevado apresentaram mais frequentemente TFG CKD-EPI basal <60 mL/min/1,73 m2. Contraste não-iônico e TFG CKD-EPI basal ≥60 mL/min/1,73 m2 foram fatores protetores à ocorrência de NIC. Em indivíduos com creatinina <1,0 mg/dL, o achado de TFG superestimada por C-G, mas não pelo CKD-EPI, foi mais frequente (sensibilidade de 100,0%; especificidade de 52,0%).
ConclusõesEm pacientes sem disfunção renal por C-G, submetidos a ICP, o achado de TFG CKD-EPI <60 ml/min/1,73 m2 implicou em maior chance de NIC, principalmente entre indivíduos do sexo masculino e de maior massa corpórea.
Contrast-induced nephropathy (CIN) is a form of acute kidney injury that occurs within a few days of exposure to iodinated contrast media, which is often used in diagnostic and therapeutic medical procedures. In the last decade, CIN has been identified as the third most common cause of renal failure acquired in the hospital environment, with important short- and long-term prognostic implications.1–5
Various factors have been identified as predictors of CIN. Individuals with pre-existing renal dysfunction are the subgroup at greatest risk for its development.6–10 Identification of such patients is thus of fundamental importance for the implementation of strategies to prevent the occurrence of CIN.2,3,10–12
A direct relationship between serum creatinine (sCr) levels and risk of developing CIN has been demonstrated, i.e. the higher the baseline sCr, the greater the likelihood of developing CIN.10 However, sCr is an imperfect marker for measuring renal function, especially because of its low sensitivity for monitoring changes in renal function, since reductions of more than 50% in glomerular filtration rate (GFR) may occur before any increase in sCr is observed. There is therefore a need for reliable methods for estimating GFR, in order to identify patients at higher risk for developing CIN.
Because of its practicality and ease of use, calculation of creatinine clearance (CrCl) is one of the most widely used methods for estimating GFR in clinical practice, most often by the Cockcroft-Gault (CG) formula, which has satisfactory reproducibility and accuracy.13,14 The variables it takes into account are weight, gender, age and sCr. However, though easy to remember and apply, the CG formula is less accurate when used in certain clinical contexts or specific populations, especially the obese and the elderly.15–20
An alternative formula for estimating GFR proposed by the Modification of Diet in Renal Disease (MDRD) Study Group considers six variables: sCr, age, ethnicity, gender, and levels of blood urea nitrogen and serum albumin. However, this tool has been validated only for patients with chronic kidney disease, and is not applicable in the context of acute kidney injury. The MDRD formula often underestimates GFR in patients with actual measured GFR greater than 60 ml/min/1.73 m2. Since it does not adjust for body mass, it also underestimates GFR for obese individuals and overestimates it in underweight individuals, compared to the CG formula.17,18,21–23
These limitations are of considerable practical importance, since measures to prevent CIN are not routinely applied in patients without significant renal dysfunction (stages 1 and 2 chronic kidney disease). However, it is not uncommon for patients without significant baseline renal dysfunction (estimated GFR ≥60 ml/min/1.73 m2 according to the CG and MDRD formulas) to develop CIN.24
In 2009, Levey et al. proposed the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, a new tool for estimating GFR, which demonstrated better performance, with greater accuracy and fewer errors in estimating GFR compared to the CG and MDRD formulas, especially for GFR >60 ml/min/1.73 m2. This formula has been validated in various populations, ethnic groups, comorbidities and clinical contexts, and both genders.15–17,20,21,26–33
In view of the above, in this study we aimed to assess the discriminatory power of the CKD-EPI formula for predicting the occurrence of CIN in patients without significant renal dysfunction (GFR ≥60 ml/min/1.73 m2) according to the CG formula after percutaneous coronary intervention (PCI).
MethodsStudy design and patient selectionThis was an observational descriptive and analytical study that retrospectively analyzed the hospital records of a historical cohort of consecutive patients, without significant baseline renal dysfunction according to the CG formula, who underwent PCI between February 2008 and August 2013 at the Dante Pazzanese Institute of Cardiology (IDPC) and who developed CIN.
CrCl estimated by the CG formula is calculated in all patients at our institution prior to diagnostic or therapeutic procedures, in order to identify individuals at greater risk for developing CIN in whom preventive measures should be implemented. For all patients included in this study, GFR was calculated retrospectively using the CKD-EPI formula before and after PCI, and CrCl was also estimated using the CG formula after PCI.
All the included patients had baseline CrCl ≥60 ml/min as estimated by the CG formula. Participants were divided into two groups: a control group with no significant baseline renal dysfunction according to the CG formula and who did not develop CIN, and another group without renal dysfunction according to CG, but who did develop CIN.
Patients for whom preprocedural sCr measurement was not available, or who were discharged on the same day as PCI and for whom sCr was not measured on subsequent days, thus making it impossible to determine the occurrence of CIN, were excluded.
Between February 2008 and August 2013, 9249 PCI procedures were performed at our institution. Of these, 8046 (86.9%) had baseline sCr measurement, enabling calculation of CrCl using the CG formula. According to the database records, there were 123 patients without significant renal dysfunction who progressed to acute kidney injury after PCI.
As the number of individuals in the control group far exceeded those who developed CIN, we calculated a minimum number of participants for this group that could be statistically representative of the study population. Based on the variability in GFR (using both CG and CKD-EPI formulas) observed among all patients without significant baseline renal dysfunction according to the CG formula, and who did not develop post-PCI CIN, a minimum sample of 12 individuals was calculated for this purpose. Finally, in order to make up the control group, we included the first case in each month during the study period with CG GFR ≥60 ml/min/1.73 m2 that did not progress to CIN. The control group accordingly consisted of 67 individuals.
Data collectionA protocol was developed to collect relevant demographic, clinical and laboratory data, pre- and post-PCI, using a standardized, semi-closed questionnaire. This information was obtained by checking the electronic database of the Invasive Cardiology Department of the IDPC. When deemed necessary by the authors, the data were confirmed by analysis of patients’ medical records.
DefinitionsCrCl estimated using the CG formula was calculated as described by Cockcroft and Gault.14 The CKD-EPI formula used was that originally described by Levey et al.25
CIN was defined as an acute decrease in renal function, not explained by causes other than exposure to contrast media, characterized by an absolute increase of ≥0.5 mg/dl in sCr or a fall of ≥25% in GFR, estimated by either of the applied formulas.34
Statistical analysisThe descriptive and analytical statistical treatment of the data obtained was performed with IBM® SPSS version 21.0.0.0®. Continuous variables were expressed as mean ± standard deviation, while categorical variables were expressed as number and percentage. The statistical significance of the intergroup difference for the same variable was determined by the chi-square test or Fisher's exact test for categorical variables and the Mann-Whitney test for continuous variables. The significance level adopted was 5.0%. Linear regression analysis was used to determine the correlation coefficients between the values obtained by the CG and CKD-EPI formulas. Bayesian analysis of the measurements obtained by the two formulas included estimates of the area under the receiver operating characteristic (ROC) curve, and identification of optimal cutoff points to predict the occurrence of CIN and their associated sensitivity and specificity. The modified Swets classification was used to classify the diagnostic efficiency of the CG and CKD-EPI formulas according to the values under the ROC curve as low (<70%), moderate (between 70% and 90%) and high (>90%). Multivariate logistic regression analysis was performed on an exploratory basis for the definition of predictors of CIN and reclassification of GFR using CKD-EPI.
ResultsAfter case-by-case analysis and precise application of the CIN definition, 76 patients were eligible and comprised the CIN group. The control group, which initially included 67 individuals, had three cases excluded after a discrepancy was found between the sCr values recorded in the database and those reported by the laboratory, and therefore totaled 64 patients (Figure 1).
There were no statistically significant differences between patients who developed CIN and those in the control group regarding age, gender, race or body mass index (BMI) (Table 1). With regard to comorbidities, acute coronary syndrome and pre-existing coronary artery disease were more frequent in the CIN group, as was use of statins, beta-blockers and calcium channel blockers.
Demographic and clinical characteristics of the study population, according to development of contrast-induced nephropathy.
| CIN (n=76) | Controls (n=64) | p | |
|---|---|---|---|
| Age, years (mean ± SD) | 61.75±9.70 | 60.55±8.42 | 0.439 |
| Gender | 0.639 | ||
| Male | 55 (72.4%) | 44 (68.8%) | |
| Female | 21 (27.6%) | 20 (31.3%) | |
| Race | 0.062 | ||
| Black | 5 (6.6%) | 0 | |
| Non-black | 71 (93.4%) | 64 (100.0%) | |
| BMI (kg/m2) | 28.9± 5.1 | 28.1±4.8 | 0.505 |
| <20 (underweight) | 0 | 1 (1.6%) | |
| ≥20 and <25 (normal) | 19 (25%) | 14 (21.9%) | |
| ≥25 and <30 (overweight) | 30 (39.5%) | 27 (42.2%) | |
| ≥30 (obese) | 27 (35.5%) | 22 (34.4%) | |
| MS | 8 (10.5%) | 7 (10.9%) | 0.937 |
| Diabetes | 31 (40.8%) | 25 (39.1%) | 0.835 |
| Hypertension | 56 (73.7%) | 55 (85.9%) | 0.074 |
| Smoker | 14 (18.4%) | 12 (18.8%) | 0.960 |
| Ex-smoker | 18 (23.7%) | 18 (28.1%) | 0.549 |
| Dyslipidemia | 47 (61.8%) | 49 (76.6%) | 0.061 |
| Family history of CAD | 2 (2.6%) | 1 (1.6%) | 1.000 |
| Known CAD | 31 (40.8%) | 41 (64.1%) | 0.006 |
| Heart failure | 2 (2.6%) | 2 (3.1%) | 1.000 |
| ACS | 34 (44.73%) | 3 (4.68%) | <0.001 |
| Medications | |||
| Aspirin | 69 (90.78%) | 60 (93.75%) | 0.516 |
| Statin | 59 (77.63%) | 58 (90.62%) | 0.038 |
| Diuretic | 11 (14.47%) | 11 (17.18%) | 0.660 |
| ACEi or ARB | 59 (77.63%) | 52 (81.25%) | 0.598 |
| Beta-blocker | 44 (57.89%) | 50 (78.12%) | 0.011 |
| CCB | 4 (5.26%) | 16 (25%) | <0.001 |
| Thienopyridine | 56 (73.68%) | 51 (79.68%) | 0.404 |
| Pre-PCI creatinine (mg/dl) | 1.00±0.19 | 0.89±0.20 | <0.001 |
| Pre-PCI GFR – CG (ml/min/1.73 m2) | 87.72±21.49 | 94.53±24.66 | 0.082 |
| Pre-PCI GFR – CKD-EPI (ml/min/1.73 m2) | 76.35±15.24 | 85.1±15.22 | <0.001 |
| Post-PCI creatinine (mg/dl) | 1.81±0.67 | 0.88±0.21 | <0.001 |
| Post-PCI GFR – CG (ml/min/1.73 m2) | 49.72±16.83 | 96.27±27.88 | <0.001 |
| Post-PCI GFR – CKD-EPI (ml/min/1.73 m2) | 41.19±12.86 | 86.01±15.94 | <0.001 |
| Type of contrast | <0.001 | ||
| Ionic, high osmolality | 20 (26.31%) | 0 | |
| Ionic, low osmolality | 51 (67.1%) | 64 (100%) | |
| Nonionic, low osmolality | 5 (6.6%) | 0 | |
| Contrast volume (ml) | 106.55±67.64 | 83.13±46.95 | 0.007 |
| Major bleeding (GUSTO criteria) | 6 (7.9%) | 1 (1.6%) | 0.125 |
| Death | 13 (17.1%) | 0 | <0.001 |
| Hospital stay (days) | 5.71±4.99 | 2.17±0.55 | <0.001 |
ACEi: angiotensin-converting enzyme inhibitor; ACS: acute coronary syndrome; ARB: angiotensin receptor blocker; BMI: body mass index; CAD: coronary artery disease; CCB: calcium channel blocker; CG: Cockcroft-Gault formula; CIN: contrast-induced nephropathy; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration formula; GFR: glomerular filtration rate; GUSTO: Global Use of Strategies to Open Occluded Arteries; MS: metabolic syndrome; PCI: percutaneous coronary intervention; SD: standard deviation.
More nephrotoxic contrast agents were used more often, and the volume of contrast agent administered was greater, in the CIN group.
Patients in the CIN group had higher mean sCr than the control group, but there was no statistical difference in the baseline GFR estimate using the CG formula. In general, the CKD-EPI formula produced lower GFR values than those provided by the CG formula, regardless of the patient group (CIN or control) or the observation period (pre- or post-PCI). In patients without significant baseline renal dysfunction according to the CG formula, application of the CKD-EPI formula to baseline serum creatinine values led to a reclassification of GFR to levels lower than 60 ml/min/1.73 m2 in 13 (9.2%) individuals, 11 (14.5%) in the CIN group and two (3.1%) in the control group (p=0.03). Male gender and high BMI were associated with GFR levels <60 ml/min/1.73 m2 after application of the CKD-EPI formula (p=0.007) (Table 2). In both groups, post-procedure GFR was also lower when calculated using the CKD-EPI formula than using the CG formula (Table 1).
Demographic and clinical characteristics of the study population, according to the presence of significant renal impairment using the Chronic Kidney Disease Epidemiology Collaboration formula.
| CKD-EPI <60 ml/min (n=13) | CKD-EPI ≥60 ml/min (n=127) | p | |
|---|---|---|---|
| Age, years (mean ± SD) | 62.77±8.24 | 61.04±9.22 | 0.341 |
| Gender | 0.007 | ||
| Male | 5 (38.5%) | 94 (74.0%) | |
| Female | 8 (61.5%) | 33 (26.0%) | |
| Race | 0.068 | ||
| Black | 2 (15.4%) | 3 (2.4%) | |
| Non-black | 11 (84.6%) | 124 (97.6%) | |
| BMI (kg/m2) | 34.12±4.76 | 28.03±4.66 | <0.001 |
| <20 (underweight) | 0 | 1 (0.8%) | |
| ≥20 and <25 (normal) | 0 | 33 (26.0%) | |
| ≥25 and <30 (overweight) | 3 (23.1%) | 54 (42.5%) | |
| ≥30 (obese) | 10 (76.9%) | 39 (30.7%) | |
| Medications | |||
| Aspirin | 12 (92.3%) | 117 (92.1%) | 0.981 |
| Statin | 9 (69.2%) | 108 (85.0%) | 0.142 |
| Diuretic | 3 (23.1%) | 19 (15.0%) | 0.430 |
| ACEi or ARB | 11 (84.6%) | 100 (78.7%) | 0.618 |
| Beta-blocker | 9 (69.23%) | 85 (66.9%) | 0.866 |
| CCB | 3 (23.1%) | 17 (13.4%) | 0.398 |
| Thienopyridine | 7 (53.8%) | 100 (78.7%) | 0.043 |
| Pre-PCI creatinine (mg/dl) | 1.23±0.21 | 0.92±0.18 | <0.001 |
| Pre-PCI GFR – CG (ml/min/1.73 m2) | 78.57±9.58 | 92.09±23.79 | 0.041 |
| Pre-PCI GFR – CKD-EPI (ml/min/1.73 m2) | 54.20±4.88 | 83.02±13.97 | <0.001 |
| Post-PCI creatinine (mg/dl) | 2.06±0.89 | 1.32±0.63 | <0.001 |
| Post-PCI GFR – CG (ml/min/1.73 m2) | 47.12±11.76 | 73.45±32.81 | 0.001 |
| Post-PCI GFR – CKD-EPI (ml/min/1.73 m2) | 33.23±11.11 | 64.59±25.99 | <0.001 |
| CIN | 11 (84.6%) | 65 (51.1%) | 0.021 |
| MS | 0 | 15 (11.8%) | 0.360 |
| Diabetes | 7 (53.8%) | 49 (38.6%) | 0.284 |
| Hypertension | 12 (92.3%) | 99 (78.0%) | 0.223 |
| Smoker | 1 (7.7%) | 25 (19.7%) | 0.461 |
| Ex-smoker | 2 (15.4%) | 34 (26.8%) | 0.514 |
| Dyslipidemia | 10 (76.9%) | 86 (67.7%) | 0.495 |
| Family history of CAD | 0 | 3 (2.4%) | 1.000 |
| Known CAD | 4 (30.8%) | 68 (53.5%) | 0.149 |
| Heart failure | 0 | 4 (3.1%) | 1.000 |
| ACS | 5 (38.5%) | 32 (25.2%) | 0.301 |
| Type of contrast | 0.520 | ||
| Ionic, high-osmolality | 1 (7.7%) | 19 (15.0%) | |
| Ionic, low osmolality | 11 (84.6%) | 104 (81.9) | |
| Nonionic, low osmolality | 1 (7.7%) | 4 (2.1%) | |
| Contrast volume (ml) | 110.76±48.03 | 94.31±61.09 | 0.104 |
| Major bleeding (GUSTO criteria) | 0 | 7 (5.5%) | 0.385 |
| Death | 9 (69.2%) | 4 (3.1%) | <0.001 |
| Hospital stay (days) | 4.23±2.35 | 4.08±4.23 | 0.091 |
ACEi: angiotensin-converting enzyme inhibitor; ACS: acute coronary syndrome; ARB: angiotensin receptor blocker; BMI: body mass index; CAD: coronary artery disease; CCB: calcium channel blocker; CG: Cockcroft-Gault formula; CIN: contrast-induced nephropathy; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration formula; GFR: glomerular filtration rate; GUSTO: Global Use of Strategies to Open Occluded Arteries; MS: metabolic syndrome; PCI: percutaneous coronary intervention; SD: standard deviation.
Patients who developed CIN had worse in-hospital outcomes than those in the control group, with longer hospital stay and higher mortality.
Patients with baseline GFR <60 ml/min/1.73 m2 according to the CKD-EPI formula presented with increased sCr levels and decreased GFR after the procedure. In this subgroup, 69.2% of the patients died, compared to only 3.1% of controls with baseline GFR ≥60 ml/min/1.73 m2 according to the CKD-EPI formula (p<0.001).
Multivariate regression showed that use of a nonionic contrast agent and baseline CKD-EPI GFR ≥60 ml/min/1.73 m2 were protective factors for CIN. Acute coronary syndrome and greater contrast volume were associated with a higher risk of post-procedure acute renal dysfunction (Table 3).
Predictors of contrast-induced nephropathy in patients with glomerular filtration rate >60 ml/min/1.73 m2 using the Cockcroft-Gault formula, according to multivariate analysis.
| OR | 95% CI | p | |
|---|---|---|---|
| Known CAD | 0.43 | 0.16-1.11 | 0.084177 |
| ACSa | 63.46 | 13.64-477.94 | <0.001 |
| Nonionic contrasta | 0.03 | 0.006-0.16 | <0.001 |
| Contrast volumea | 1.01 | 1.001-1.020 | 0.03 |
| Pre-PCI CG GFR | 0.99 | 0.97-1.02 | 0.80 |
| Pre-PCI CKD-EPI GFR ≥60 ml/min/1.73 m2a | 0.95 | 0.92-0.98 | 0.013220 |
Statistically significant variables.
ACS: acute coronary syndrome; CAD: coronary artery disease; CG: Cockcroft-Gault formula; CI: confidence interval; CIN: contrast-induced nephropathy; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration formula; GFR: glomerular filtration rate; OR: odds ratio.
Male gender, body weight, BMI and black race were predictors of lower GFR when calculated using the CKD-EPI formula in individuals without significant basal renal dysfunction according to the CG formula. When multivariate regression was applied, only body weight and male gender were confirmed predictors of baseline GFR <60 ml/min/1.73 m2 according to the CKD-EPI formula (Table 4). BMI is not related to lower GFR calculated by the CKD-EPI formula, since, according to the principle of collinearity, it correlates closely with body weight and is influenced by it.
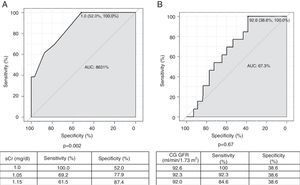
Two ROC curves were plotted using baseline sCr and baseline GFR according to the CG formula as the dependent and continuous variables, projecting cutoff values from which baseline GFR <60 ml/min/1.73 m2 with the CKD-EPI formula can be found (Figure 2). Baseline GFR according to the CG formula was not significantly associated with baseline CKD-EPI GFR <60 ml/min/1.73 m2. Considering preprocedural sCr values, a cutoff point of 1.0 mg/dl has a sensitivity of 100% and specificity of 52.0% to find baseline CKD-EPI GFR <60 ml/min/1.73 m2 (p=0.002). For intermediate values of sensitivity and specificity (69.2% and 77.9%, respectively), a cutoff sCr value of 1.05 mg/dl is more appropriate.
Receiver operating characteristic curves for serum creatinine (A) and glomerular filtration rate (GFR) estimated by the Cockcroft-Gault formula (B), as a function of GFR <60 ml/min estimated by the Chronic Kidney Disease Epidemiology Collaboration formula. AUC: area under the curve; CG: Cockcroft-Gault formula; CIN: contrast-induced nephropathy; CrCl: creatinine clearance; PCI: percutaneous coronary intervention; sCr: serum creatinine.
Routine analysis of renal function is an essential part of clinical assessment prior to PCI or any medical procedure using iodinated contrast agents. GFR is considered to be the most reliable marker of renal function, whether or not a pathological condition is present.25 Since this index is not easy to measure, in clinical practice it is estimated using formulas such as the CG, MDRD and CKD-EPI. Mathematically, these formulas differ in the way they combine their variables, such as sCr, age, race, gender, body weight, and body surface area. In practice, each of these formulas has been validated in various specific populations, so that when used correctly it will be appropriate for the clinical context in question.16,23,28,29,31,35,36
The presence of prior renal dysfunction (estimated GFR <60 ml/min/1.73 m2) is the most important predictor of CIN after PCI. However, clinical practice shows that, even in populations without significant renal dysfunction, acute kidney injury is not uncommon after the procedure. Chong et al.24 demonstrated that age, female gender, insulin-dependent diabetes, hypotension, anemia, and left ventricular systolic dysfunction are important predictors of CIN in individuals with normal sCr. Currently, a significant proportion of procedures in interventional cardiology departments are performed electively on an outpatient basis,37,38 and thus the individual's complete medical history is often unavailable, which makes it difficult to identify these predictors at admission pre-procedure. There is therefore a need to improve assessment of these patients.
In this study, we found that 9.2% of patients without baseline renal dysfunction calculated using the CG formula actually already showed reduced GFR when the CKD-EPI formula was applied. Moreover, 84.6% in this subgroup developed CIN. Logistic regression analysis showed that for patients in whom the CG formula may have overestimated GFR, the finding of GFR <60 ml/min/1.73 m2 using the CKD-EPI formula was an independent predictor of progression to acute kidney injury. In our sample, other classic risk factors for CIN, independent of previous renal function, were confirmed, such as acute coronary syndrome and the type and volume of contrast agent used. The absence of other classical factors for CIN in the present analysis is due to various factors, especially the small sample size (mainly in terms of age and prevalence of diabetes) and incomplete information in the database, including serum hemoglobin levels and left ventricular ejection fraction.39–41
Of particular note is the fact that GFR calculated by the CKD-EPI formula was lower in absolute terms in males and in those with greater body weight. Regarding weight, the mathematical impact of this variable on estimation of GFR by the CKD-EPI formula is less than when the CG formula is applied. Therefore, the latter is more likely to underestimate GFR in obese and overweight individuals, who were in the majority in our sample. With regard to gender, the CG formula uses a value of 0.85 for females as a correction factor. In the CKD-EPI formula, the figures of 0.9 for men and 0.7 for women are used to correct for gender, both with a negative power exponent, which directly and proportionally influences estimation of GFR.13,15,25,42
Although the frequency of use of different categories of contrast media differed significantly among individuals who did or did not develop CIN, this was not the case when patients were categorized according to presence of prior renal dysfunction using the CKD-EPI formula. This was confirmed in multivariate analysis, in which reclassification of pre-PCI renal function was confirmed as a predictor of progression to CIN, independently of the contrast agent used.
Additionally, analysis of the ROC curve shows that sCr values less than 1.0 mg/dl have a sensitivity of 100% for a finding of CKD-EPI GFR <60 ml/min/1.73 m2, but with intermediate specificity. Thus, since CIN prevention requires optimal screening of at-risk populations, an sCr value of 1.0 mg/dl represents the ideal cutoff for this variable, with excellent sensitivity and intermediate specificity, when the CKD-EPI formula is applied, in conjunction with or instead of the CG formula, in the assessment of patients prior to PCI.
Study limitationsOne of the limitations of this study is that, regardless of the formula used, the body weight used in the GFR calculation was the actual weight, not that predicted for height and age. In addition, the formulas were not compared with laboratory GFR measurements, so it cannot be positively stated that the CKD-EPI formula is more sensitive than the CG formula for identifying GFR <60 ml/min/1.73 m2. In addition, GFR estimates, regardless of the formula used, may be inaccurate when sCr levels are not stable, as may occur after exposure to an iodinated contrast agent. GFR estimates in this context are merely an extrapolation, which is far from ideal. Finally, the information used in the analyses was mainly extracted from an electronic database. This approach is subject to the biases specific to this type of data collection, under-recording of non-negligible variables being the most common.
Given the retrospective nature of this analysis, there is a need for a prospective, randomized study that estimates GFR with both the CG and CKD-EPI formulas and compares the effect of prophylactic measures for CIN in individuals with GFR overestimated by the former formula, in particular hydration with isotonic saline before and after exposure to the contrast agent.
ConclusionsSince the development of CIN has important prognostic implications, including increased mortality, accurate stratification of individuals at increased risk for this condition is essential.
Application of the CKD-EPI formula for assessing GFR at the time of pre-PCI assessment, instead of or even jointly with the CG formula, plays an important role in the prevention of CIN. In particular, individuals with sCr less than 1.0 mg/dl, males, obese individuals, and those undergoing the procedure due to acute coronary syndrome, may benefit more from this approach.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Nunes MBG, Filho AC, Alvares VRC, et al. Fórmula de CKD-EPI versus Cockcroft-Gault na predição de nefropatia induzida por contraste após intervenção coronária percutânea, em pacientes sem disfunção renal significativa. Rev Port Cardiol. 2018;37:25–33.