Pulmonary endarterectomy (PEA) is a potentially curative procedure in patients with chronic thromboembolic pulmonary hypertension (CTEPH). This study reports the initial experience of a Portuguese PH center with patients undergoing PEA at an international surgical reference center.
MethodsProspective observational study of consecutive CTEPH patients followed at a national PH center, who underwent PEA at an international surgical reference center between October 2015 and March 2019. Clinical, functional, laboratory, imaging and hemodynamic parameters were obtained in the 12 months preceding the surgery and repeated between four and six months after PEA.
Results27 consecutive patients (59% female) with a median age of 60 (49-71) years underwent PEA. During a median follow‐up of 34 (21-48) months, there was an improvement in functional class in all patients, with only one cardiac death. From a hemodynamic perspective, there was a reduction in mean pulmonary artery pressure from 48 (42-59) mmHg to 26 (22-38) mmHg, an increase in cardiac output from 3.3 (2.9-4.0) L/min to 4.9 (4.2-5.5) L/min and a reduction in pulmonary vascular resistance from 12.1 (7.2-15.5) uW to 3.5 (2.6-5,2) uW. During the follow‐up, 44% (n=12) of patients had no PH criteria, 44% (n=12) had residual PH and 11% (n=3) had PH recurrence. There was a reduction of N‐terminal pro‐B‐type natriureticpeptide from 868 (212-1730) pg/mL to 171 (98-382) pg/mL. Rright ventricular systolic function parameters revealed an improvement in longitudinal systolic excursion and peak velocity of the plane of the tricuspid ring from 14 (13–14) mm and 9 (8–10) cm/s to 17 (16–18) mm and 13 (11-15) cm/s, respectively. Of the 26 patients with preoperative right ventricular dysfunction, 85% (n=22) recovered. The proportion of patients on specific vasodilator therapy decreased from 93% to 44% (p<0.001) and the proportion of those requiring oxygen therapy decreased from 52% to 26% (p=0.003). The six‐minute walk test distance increased by about 25% compared to the baseline and only eight patients had significant desaturation during the test.
ConclusionPulmonary endarterectomy performed at an experienced high-volume center is a safe procedure with a very favorable medium-term impact on functional, hemodynamic and right ventricular function parameters in CTEPH patients with operable disease. It is possible for PH centers without PEA differentiation to refer patients safely and effectively to an international surgical center in which air transport is necessary.
A tromboendarterectomia pulmonar (TP) é um procedimento potencialmente curativo em doentes com hipertensão pulmonar (HP) tromboembólica crónica (TEC). O objetivo deste trabalho é reportar a experiência inicial de um centro português de tratamento de HP em doentes submetidos a TP num centro de referência cirúrgico internacional.
MétodosEstudo observacional prospetivo de doentes consecutivos com diagnóstico de CTEPH seguidos em centro nacional de tratamento de HP e submetidos a TP em centro de referência cirúrgico internacional entre outubro de 2015 e março de 2019. Parâmetros clínicos, funcionais, laboratoriais, imagiológicos e hemodinâmicos foram obtidos nos 12 meses precedentes à cirurgia e repetidos entre quatro a seis meses após a TP.
ResultadosForam submetidos a TP 27 doentes consecutivos (59% do sexo feminino) com mediana de 60 (49-71) anos. Durante um seguimento mediano de 34 (21-48) meses, verificou‐se melhoria da classe funcional em todos os doentes, tendo ocorrido apenas um óbito de causa cardíaca. Do ponto de vista hemodinâmico, observou‐se redução da pressão média na artéria pulmonar de 48 (42-59) mmHg para 26 (22-38) mmHg, aumento do débito cardíaco de 3,3 (2,9-4,0) L/min para 4,9 (4,2-5,5) L/min e redução das resistências vasculares pulmonares de 12,1 (7,2-15,5) uW para 3,5 (2,6-5,2) uW. Tendo em conta os parâmetros hemodinâmicos avaliados pós‐TP e a sua evolução durante o seguimento clínico, 44% (n = 12) dos doentes deixaram de ter critérios de HP, 44% (n = 12) mantiveram HP e 11% (n = 3) evoluíram com recorrência de HP. Laboratorialmente, a salientar redução do NT‐proBNP de 868 (212–1730) pg/mL para 171 (98–382) pg/mL. Dos parâmetros de função sistólica ventricular direita, verificou‐se melhoria da excursão e velocidade de pico sistólicas longitudinais do plano do anel tricúspide de 14 (13-14) mm e 9 (8-10) cm/s para 17 (16-18) mm e 13 (11-15) cm/s, respetivamente. Dos 26 doentes com critérios de disfunção sistólica ventricular direita pré‐cirurgia, 85% (n = 22) apresentaram critérios de recuperação. A proporção de doentes sob terapêutica vasodilatadora específica diminuiu de 93% para 44% (p < 0,001) e a proporção daqueles requerendo OLD diminuiu de 52% para 26% (p = 0,003). A distância percorrida no teste dos seis minutos de marcha aumentou em cerca de 25% relativamente ao valor prévio à intervenção cirúrgica e apenas oito doentes mantiveram dessaturação significativa durante a prova.
ConclusãoA TP realizada em centro cirúrgico de elevado volume é um procedimento seguro e com impacto muito favorável em médio prazo nos parâmetros funcionais, hemodinâmicos e de função ventricular direita em doentes com HPTEC operável. É possível, para centros de tratamento de HP sem diferenciação em TP, a referenciação dos doentes com segurança e efetividade a um centro cirúrgico internacional em que para tal seja necessário aerotransporte.
Chronic thromboembolic pulmonary hypertension (CTEPH) is in the group 4 pulmonary hypertension (PH) clinical classification1, alongside other forms of vascular obstruction. It is defined in the presence of a precapillary hemodynamic phenotype resulting from incomplete resolution and fibrotic transformation of thromboembolic material in the pulmonary arteries after at least three months of effective anticoagulation. Its etiopathogenesis is likely to be one or more episodes of acute pulmonary embolism (PE), and possible phenomena of in situ pulmonary thrombosis may also be considered. The correct identification of the nosological entity, which is often underdiagnosed, is of utmost importance, given the curative potential of treatment. Historically, the long-term prognosis of this disease has been poor, with an estimated five-year survival rate in the absence of adequate treatment of about 10%2. Pulmonary endarterectomy (PEA) has emerged as the gold standard approach to CTEPH in patients with a surgically accessible fibro-thromboembolic burden3, leading to proven symptomatic and prognostic benefits4. It should be noted that both the imaging techniques for defining operability and the surgical intervention process should be carried out at centers with proven experience in the diagnosis and treatment of PH5,6 with operative mortality rates <5% and significant improvement in symptoms, functional capacity and hemodynamic parameters7–9.
This study aims to report the initial experience of a Portuguese center for the treatment of PH analyzing medium-term survival and functional, hemodynamic, and right ventricular function results in patients undergoing PEA.
MethodsStudy design and patient selectionProspective observational study of consecutive patients diagnosed with CTEPH followed at a national HP treatment center and undergoing PEA at an international surgical referral center — Royal Papworth Hospital NHS Foundation Trust (Cambridge) between October 2015 and March 2019.
The present study was conducted respecting the ethical standards of the Helsinki Agreement and was approved by the ethics committee of the local institution. All participants provided informed consent.
Chronic thromboembolic pulmonary hypertension: diagnostic algorithmThe diagnosis of CTEPH was made according to international recommendations6: precapillary PH (mean pulmonary artery pressure (mPAP) ≥25 mmHg and pulmonary capillary wedge pressure ≤15 mmHg) in the presence of chronic thromboembolic material on pulmonary angiography (invasive or computed tomography (CT)), in patients who had been under therapeutic anticoagulation for at least three months.
All patients diagnosed with symptomatic CTEPH according to the World Health Organization (WHO) functional class (FC) ≥2 were discussed in an inter-center multidisciplinary meeting to determine operability. All patients with surgically accessible thrombotic burden in lobar, segmental and/or subsegmental branches in the absence of comorbidities determining an unfavorable risk/benefit ratio for surgical intervention were considered operable, regardless of age or magnitude of elevation of pulmonary vascular resistance (PVR). An electronic request for international medical assistance was submitted to the International Patient Mobility Portal of the Directorate-General of Health for each patient with an indication for PEA, due to the lack of specialized technical resources nationally. All submitted requests were approved.
Patients deemed inoperable were advised to seek medical therapy with specific pulmonary vasodilators and referred to a balloon pulmonary angioplasty program (which started at our center in January 2018).
Surgical techniqueThe PEA procedures were performed according to previously described principles10. All patients underwent surgery using the deep hypothermic circulatory arrest technique. In some selected patients, additional procedures were performed, such as valvular surgery or myocardial revascularization.
Surgical specimens were categorized into four types according to Jamieson’s11 classification. The removed material was sent systematically for anatomopathological analysis to exclude malignancy, such as pulmonary artery sarcoma.
Pre- and post-surgery approach and assessmentClinical, functional, laboratory, imaging and hemodynamic parameters were obtained in the 12 months prior to surgery and repeated four to six months after the intervention at the national treatment center.
The six-minute walk test (6MWT) was performed according to international recommendations12.
The presence of residual PH after PEA was confirmed if mPAP ≥25 mmHg and PVR >3 uW at the first hemodynamic assessment and persistent PH if the aforementioned criteria reappeared at subsequent assessments.
In patients on specific vasodilator therapy, this was systematically stopped on the day of surgery.
In the presence of at least one cardiovascular risk factor, age >40 years (men) or postmenopausal age, chest pain, or a history of atherosclerotic disease, patients underwent coronary angiography (invasive or CT) to exclude coronary artery disease.
Patients with hypoxemia at rest, at sea level, with or without criteria for long-term oxygen therapy (LTOT) and/or ambulation were tested for air travel at the air travel appointment. As part of this assessment, a hypoxic challenge test was performed (normobaric simulation of hypoxia in the cabin of commercial aircraft), which determines the need for oxygen therapy during the trip and the rate to be prescribed. As part of the clinical travel schedule, transport to and from the airport over land was also guaranteed.
Anticoagulation was resumed post-surgery in all patients.
Intraoperative and postoperative complications and causes of mortality were recorded. Survival was assessed until the date of the patient’s last visit to the PH treatment center.
Statistical analysisCategorical variables were described according to their absolute and relative frequency and were compared using chi-square and Fisher’s exact tests. Continuous variables with normal distribution were characterized with mean and standard deviation, and compared using Student’s t-test, analysis of variance, and the paired t-test to compare pre- and post-PEA values. Continuous variables with non-normal distribution were described with median and IQR and were compared with nonparametric Mann–Whitney U and Kruskal–Wallis tests, and the paired samples Wilcoxon test was used to compare pre- and post-PEA values. The analysis was performed using SPSS software (version 26.0; SPSS Inc., Chicago, IL, USA).
ResultsPopulation and preoperative resultsDemographic and clinical characterization of the population is presented in Table 1. Twenty-nine consecutive patients with the diagnosis of CTEPH were assessed. Two patients were excluded after being considered inoperable due to the presence of a highly distal fibrothrombotic burden. A total of 27 patients (59% female) with a median age of 60 (49-71) years underwent PEA. On average, the diagnosis was established 3.3 ± 0.7 years after symptom onset and an average of 2.6 ± 1.0 years elapsed between diagnosis and PE. A previous history of acute pulmonary embolism (PE) was identified in 59% (n=16) of patients. In addition, 15% (n=4) had diagnostic criteria for13 antiphospholipid syndrome (APS) and 11% (n=3) had hereditary thrombophilias — protein S deficiency.
Demographics, risk factors and co-morbidities of the population with chronic thromboembolic pulmonary hypertension who underwent pulmonary endarterectomy.
| Frequency (N = 27) | |
| Age, years, mean ± standard deviation | 60 ± 3 |
| Female gender, n (%) | 16 (59) |
| Identified risk factors for CTEPH, N (%) | |
| History of PE/DVT | 16 (59) |
| Antiphospholipid syndrome | 4 (15) |
| Hereditary thrombophilia | 3 (11) |
| Chronic inflammatory disease | 1 (4) |
| Chronic myeloproliferative syndrome | 2 (7) |
| Neoplasm in remission | 1 (4) |
| Pacemaker | 0 (0) |
| Thyroid hormone replacement therapy | 6 (22) |
| Splenectomy | 0 (0) |
| Other comorbidities, N (%) | |
| Coronary heart diseasea | 1 (4) |
| Atrial fibrillation | 4 (15) |
| Diabetes | 2 (7) |
| Chronic kidney disease | 7 (26) |
| Anemia | 7 (26) |
| Parenchymal-interstitial lung disease with functional significanceb | 3 (11) |
CTEPH: chronic thromboembolic pulmonary hypertension; PE: pulmonary embolism; DVT: deep vein thrombosis.
At baseline, 74% (n=20) of patients were in WHO FC III and 93% (n=25) were on specific pulmonary vasodilator therapy as a bridging to surgery. All patients were on oral anticoagulation: 70% (n=19) with warfarin and 30% (n=8) with DOACs. In total, 52% (n=14) of patients were on LTOT. The distance walked in the (6MWT was 294 ± 23 m and significant peripheral desaturation was found in the test (≤88% oxygen saturation measured by pulse oximeter) in 89% (n=24) of cases.
In terms of hemodynamics, the median value of the mean right atrium pressure (mRAP) was 11 (9-16) mmHg, the mPAP was 48 (42-59) mmHg, cardiac output was 3.3 (2.9-4.0) L/min and the PVR was 12.1 (7.2-15.5) uW.
Laboratory tests showed a median N-terminal prob-B type natriuretic peptide (NT-proBNP) value of 868 (212-1730) pg/mL and a median hemoglobin value of 13.9 (12.5-15.4) g/dL. It should be noted that the patients did not present renal dysfunction, and the median serum creatinine was 0.97 (0.81-1.18) mg/dL.
Right ventricular longitudinal systolic function parameters revealed: the median values of the tricuspid annular plane systolic excursion (TAPSE) and peak systolic (S’) velocity of tricuspid annulus were 14 (13-14) mm and 9 (8-10) cm/s, respectively. Twenty-six patients had criteria for right ventricular dysfunction, defined by the presence of TAPSE <16 mm and/or S’ <11 cm/s.
With the exception of one patient, who required medical air transport due to the severity of her respiratory failure, all patients traveled to the surgical center via low-cost commercial air travel.
Operative and postoperative resultsTable 2 illustrates the surgical procedure-related characteristics. Mean cardiopulmonary bypass time was 288 ± 33 min and the median times (IQR) of aortic clamping and deep hypothermic circulatory arrest were 65 (45-84) min and 34 (23-44) min, respectively. All patients had significant bilateral thrombotic burden. Most had proximal disease (types 1 and 2), 15% (n=4) of whom had a distal component. Figure 1 illustrates samples from some of the PEA specimens.
Data relating to the surgical procedure.
| Surgical classificationa, N (%) | I | 6 (22) |
| II | 17 (63) | |
| III | 4 (15) | |
| IV | 0 (0) | |
| Aortic clamping time, min, median (IQR) | 65 (45-84) | |
| Cardiopulmonary bypass time, min, mean ± SD | 288 ± 33 | |
| Deep hypothermic circulatory rest time, min, median (IQR) | 34 (23-44) | |
| Additional surgical procedures, N (%) | ||
| Tricuspid annuloplasty ring | 2 (7) | |
| Biological mitral prosthesis | 1 (4) | |
| Closure of foramen ovale | 2 (7) | |
| Left internal mammary graft to the anterior descending artery | 1 (4) | |
| Postoperative complications, N (%) | ||
| Late reperfusion edema | 1 (4) | |
| Cardiac tamponade requiring surgical re-intervention | 2 (7) | |
| Operative wound infection | 2 (7) | |
| Postoperative VV-ECMO | 1 (4) | |
| Non-traumatic subdural hematoma | 1 (4) | |
| Acute kidney injury with temporary need for renal replacement therapy | 1 (4) | |
| De novo atrial fibrillation | 3 (11) | |
| Heparin-induced thrombocytopenia, type II | 2 (7) | |
| Surgery time — intensive care discharge (days) | 5 ± 3 | |
| Surgery time — hospital discharge (days) | 11 ± 5 | |
VV-ECMO: extra‐corporeal membrane oxygenation.
Additional surgical procedures were performed in 22% (n=6) of the patients (Table 2): two tricuspid plastic surgeries with ring placement, one bioprosthesis implantation in mitral position, two foramen ovale closures, and one left internal mammary graft to the anterior descending artery.
The anatomopathological study excluded malignancy in all surgical specimens and described the presence of organized fibrothrombotic material.
The mean length of stay of the patients in the intensive care unit was 5 ± 3 days, and they were discharged home 11 ± 5 days after PEA. Postoperative complications occurred in 19% (n=5) of patients, with full recovery during follow-up (Table 2). One patient required advanced mechanical respiratory support for five days in the form of veno-venous extracorporeal membrane oxygenation (VV-ECMO) due to extensive early reperfusion edema. The same patient had acute kidney injury — classified as stage three according to the Kidney Disease Improving Global Outcomes, requiring temporary continuous renal replacement therapy. One patient required three days of invasive mechanical ventilation 19 days after surgery, following air travel, with a diagnosis of late reperfusion edema. Two patients required surgical re-intervention for post-PEA cardiac tamponade. One patient was diagnosed with an uncomplicated subdural hematoma, which manifested as postoperative headaches. Three patients had episodes of atrial fibrillation again postoperatively, which was reversed chemically with intravenous amiodarone. Two patients required longer hospitalization due to surgical wound infection, one of them requiring application of a negative pressure wound therapy.
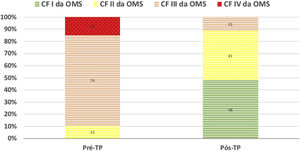
During a median follow-up of 34 (21-48) months, there was improvement in FC in all patients (Figure 2). In one case, despite the clinical improvement observed in the first two years post-PEA, there was global deterioration, and the patient died after four years due to worsening of residual PH and consequent right ventricular failure. There was no recurrence of acute or chronic pulmonary thromboembolic disease.
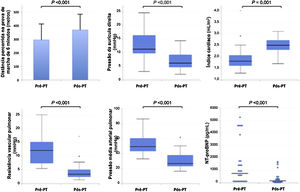
From the hemodynamic point of view, median mPAP values decreased to 26 (22-38) mmHg, CO increased to 4.9 (4.2-5.5) L/min and PVR decreased to 3.5 (2.6-5.2) uW. Considering the hemodynamic parameters assessed after PEA and their evolution during clinical follow-up, 44% (n=12) of patients no longer had criteria for PH, 44% (n=12) maintained PH and 11% (n=3) developed recurrent PH at follow-up, although it was generally less severe compared to the pre-PEA state. More specifically, MPAP decreased in 93% (n=25) of patients.
NT-proBNP decreased to 171 (98-382) pg/mL and hemoglobin to 12.8 (11.8-14.2) mg/dL. Serum creatinine levels remained at similar to those pre-PEA (0.9 (0.8-1.1) mg/dL). Figure 3 compares the functional, hemodynamic and laboratory parameters before and after PEA.
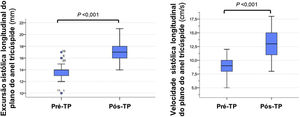
Regarding right ventricular longitudinal systolic function parameters, TAPSE increased to 17 (16-18) mm and tricuspid S’ to 13 (11-15) cm/s. Of the 26 patients with criteria for pre-surgery right ventricular systolic dysfunction, 85% (n=22) had criteria for recovery (Table 3). Figure 4 compares the echocardiographic parameters of right ventricular function before and after PEA.
Comparison of clinical status and hemodynamic and echocardiographic parameters before and after pulmonary thromboendarterectomy.
| Pre-PTE | Post-PTE | p-Value | ||
| BMI, kg/m2, median (IQR) | 28 (25-33) | 28 (22-46) | NS | |
| Functional parameters | ||||
| Functional class (WHO), N (%) | I | 0 (0) | 13 (48) | 0,022 |
| II | 3 (11) | 11 (41) | ||
| III | 20 (74) | 3 (11) | ||
| IV | 4 (15) | 0 (0) | ||
| 6MWT, m, mean ± SD | 294 ± 23 | 366 ± 23 | <0,001 | |
| Hemodynamic parameters, median (IQR) | ||||
| mRMP, mmHg | 11 (9-16) | 6 (4-10) | <0.001 | |
| mPAP mmHg | 48 (42-59) | 26 (22-38) | <0.001 | |
| CO, L/min | 3.3 (2.9-4.0) | 4.9 (4.2-5.5) | 0.001 | |
| CI, L/min/m2 | 1.8 (1.6-2.1) | 2.5 (2.3-2.7) | 0.001 | |
| PVR, uW | 12.1 (7.2-15.5) | 3.5 (2.6-5.2) | <0.001 | |
| Right ventricular function parameters, median (IQR) | ||||
| NT-proBNP, pg/mL | 868 (212-1730) | 171 (98-382) | <0.001 | |
| TAPSE, mm | 14 (13-14) | 17 (16-18) | <0.001 | |
| S’ tricuspid, cm/s | 9 (8-10) | 13 (11-15) | <0.001 | |
| Right ventricular dysfunction (TAPSE <16 mm and/or S’ tricuspid <11 cm/s), N (%) | 26 (96) | 4 (15) | <0.001 | |
| Therapeutic, N (%) | ||||
| Long-term oxygen therapy | 14 (52) | 7 (26) | 0,003 | |
| Oral anticoagulation | 27 (100) | 27 (100) | NS | |
| Warfarin | 19 (70) | 14 (52) | 0.001 | |
| Direct oral anticoagulants | 8 (30) | 13 (48) | 0.001 | |
| Specific pulmonary vasodilators | 25 (93) | 12 (44) | <0.001 | |
| PDE5i | 13 (48) | 1 (4) | <0.001 | |
| Riociguat | 11 (41) | 11 (41) | NS | |
| ERA | 12 (44) | 7 (26) | <0.001 | |
| Prostacyclin analogues | 1 (4) | 1 (4) | NS | |
BMI: body mass índex; WHO: world health organization; 6MWT: distance walked in the 6-min walk test; mRMP: mean right atrial pressure; mPAP: mean pulmonary artery pressure; CO: cardiac output; CI: cardiac index; PTE: pulmonary thromboendarterectomy; PVR: pulmonary vascular resistances; NT-proBNP: N-terminal pro-B-type peptide; TAPSE: tricuspid annular plane systolic excursion; PDE5i: phosphodiesterase type 5 inhibitors; ERA: endothelin receptor antagonists; VV-ECMO: extra‐corporeal membrane oxygenation.
The proportion of patients on specific vasodilator therapy decreased from 93% to 44% (p<0.001) and the proportion of those requiring LTOT decreased from 52% to 26% (p=0.003). The 6MWT increased to 367 ± 118 m, 25% higher than before surgery, and only eight patients maintained significant desaturation during the test (Table 4).
Predictors of residual pulmonary hypertension after pulmonary thromboendarterectomy.
| Pre-PTE variables | Residual PH post-PTE | Resolution of PH by PTE | p-Value |
|---|---|---|---|
| (N = 15) | (N = 12) | ||
| Female gender | 11 (73%) | 5 (42%) | NS (0.096) |
| Age, years, mean ± SD | 65 ± 11 | 53 ± 14 | 0,028 |
| Antiphospholipid antibody syndrome, n (%) | 2 (13%) | 2 (18%) | NS |
| Hereditary thrombophilia, n (%) | 2 (13%) | 1 (9%) | NS |
| Functional class, mean ± SD | 3.2 ± 0,6 | 2.8 ± 0.4 | NS |
| Time from onset of symptoms to diagnosis, months, median (IQR) | 25 (12-44) | 27 (18-82) | 0.107 |
| Time from diagnosis to surgery, months, median (IQR) | 23 (8-64) | 9 (3-20) | 0.025 |
| BMI, kg/m2, median (IQR) | 27.9 (24.7-32.7) | 27.5 (24.9-33.6) | NS |
| 6MWT, meters, mean ± SD | 244 ± 84 | 357 ± 127 | 0.014 |
| mRMP: mean right atrial pressure, mmHg, median (IQR) | 11 (10-18) | 11 (7-15) | NS |
| mPAP, mmHg, median (IQR) | 52 (42-60) | 47 (39-52) | NS |
| CO (L/min), median (IQR) | 3.2 (2,8-3,5) | 3,9 (3,2-4,8) | NS (0.067) |
| CI, L/min/m2, median (IQR) | 1.7 (1,6-1,9) | 2,0 (1,7-2,2) | NS |
| RVP, uW, median (IQR) | 13.4 (10,6-18,4) | 9,2 (6,9-13,1) | NS (0.059) |
| Resting heart rate (bpm), mean ± SD | 83 ± 10 | 73 ± 12 | 0.032 |
| Hemoglobin (g/dL), mean ± SD | 13.1 ± 21 | 14,8 ± 1,9 | 0.021 |
| NT-proBNP (pg/mL) | 1065 (267-4451) | 382 (82-1433) | NS |
| TAPSE (mm), mean ± SD | |||
| S’ tricuspid (cm/s), mean ± SD | |||
| Long-term oxygen therapy | 11 (73%) | 3 (25%) | 0.013 |
| Treatment with a specific pulmonary vasodilator | 15 (100%) | 10 (83%) | NS |
| Number of associated pulmonary vasodilator drugs | 1.5 ± 0.6 | 1.3 ± 0.9 | NS |
BMI: body mass index; WHO: world health organization; 6MWT: distance walked in the 6-min walk test; mRMP: mean right atrial pressure; mPAP: mean pulmonary artery pressure; CO: cardiac output; CI: cardiac index; PTE: pulmonary thromboendarterectomy; PVR: pulmonary vascular resistances; NT-proBNP: N-terminal pro-B-type peptide; TAPSE: tricuspid annular plane systolic excursion; PDE5i: phosphodiesterase type 5 inhibitors; ERA: endothelin receptor antagonists.
For the first time in Portugal, this prospective observational study describes the initial experience of patients with CTEPH followed at a national center (Portugal) for the treatment of PH undergoing PEA at an international reference surgical center (United Kingdom).
This study attested to the safety and efficacy, in terms of survival and clinical and hemodynamic improvement, of PEA performed at a high-volume surgical center. Of the three specific treatments for CTEPH, PEA is the one with the highest evidence of efficacy5,14–16, with a class of recommendation I, level of evidence C, in operable patients6. European recommendations and the 6th World Symposium on Pulmonary Hypertension differentiating between operable and non-operable CPTH. However, in clinical practice this distinction is subjective and determined by local surgical experience and practice. Since the definition of operability is a major factor in the assessment of patients with CTEPH, it should be reached within multidisciplinary input at centers with proven experience.
In the largest published study with patients undergoing PEA, mPAP was reduced from 46 mmHg to 26 mmHg and PVR from 9.0 uW to 3.2 uW8. The magnitude of hemodynamic improvement is very similar to that reported in the present study and at the center where patients were operated. This study achieved the largest long-term follow-up series, attesting to the excellent post-PEA survival of patients with CTEPH4. In that study, residual PH (mPAP ≥25 mmHg at six months after PEA) was a frequent finding at 51%, a rate similar to that of our series (56% (n=15)). Persistent PH after surgery has been reported in 11% to 35% of patients in other series, with important prognostic implications15–20. It should be noted, however, that there is still no unanimity regarding the definition of residual PH after PEA, either in terms of absolute MPAP values or in terms of the timing of assessment. In our study, factors associated with the persistent PH after surgery were longer delay from the onset of symptoms to diagnosis and from diagnosis to PEA, older age, and severity of respiratory failure. This highlights the importance of a timely diagnosis and intervention, both of which are essential to reduce the development and progression of vasculopathy in areas without fibrothrombotic burden (“reactive” or “secondary” arteriopathy).
Even though a significant number of patients maintained the hemodynamic criteria for PH after surgery, there was a significant improvement in terms of functional capacity. In a large international prospective registry of CPEAPH, 6MWT increased from 362 to 459 m in 386 operated patients9. In long-term follow-up of 880 operated patients in the United Kingdom, 6MWT increased from 260 to 353 m and WHO FC (% I/II/III/IV) improved from 0/9/68/23 to 34/47/15/04. In our experience, we found a similar improvement in functional capacity after PEA as in the aforementioned studies: 6MWT increased from 294 to 366 m and WHO FC (% I/II/III/IV) improved from 0/11/74/15 to 48/41/11/0 (Figure 2).
Despite the undeniable efficacy of PEA in patients with CTEPH, the procedure is complex and is associated with variable in-hospital mortality rates, which can reach 16%18,21,22. Accumulated experience and the tendency to concentrate the procedure in high-volume centers have resulted in a proven significant reduction in in-hospital mortality, which lies at <2.5%, identical to the reference center for the patients included in this study4. Results from the largest prospective international CTEPH registry show that in centers with less than ten procedures per year, in-hospital and long-term mortality rates are double those of high-volume centers23. In our study, there was no mortality. During follow-up, there was one death related to disease progression, despite initial improvement after surgery. This patient’s variables were considered independent predictors of long-term mortality: poor functional capacity, high mPAP, mRAP and PVR, low cardiac index and the need for early initiation of pulmonary vasodilator therapy after surgery24,25. In the international prospective registry of CTEPH, perioperative complications were recorded in 49.2% of cases, a higher rate than that observed in our patient population, despite the small sample size. The complications reported in this analysis are unanimously found in other series. One case of non-traumatic subdural hematoma was documented, a complication recently described as underdiagnosed in patients undergoing PEA26.
Regarding a key point of surgery — the deep hypothermic circulatory time, sometimes requiring a subtle compromise between adequate endarterectomy versus postoperative morbidity. An increase in circulatory arrest time is associated with an increased incidence of neurological complications27 but a greater reduction in PVR21,22. In the international prospective registry of CTEPH, the median duration of circulatory arrest was only 35 minutes9, similar to our population — 34 minutes.
Chronic thromboembolic pulmonary hypertension has been described according to a tri-compartmental disease model: 1) a component of proximal vascular disease that can be approached via PEA; 2) a component of subsegmental thrombotic obstructive disease sometimes not surgically accessible and a potential target for pulmonary balloon angioplasty28,29; 3) a component of small vessel vasculopathy, histologically similar to pulmonary arterial hypertension, resulting from blood hyperflow in areas without fibrothrombotic obstruction and the main target of medical vasodilator therapy30,31. In our series, most (85%) patients had proximal disease (types I and II — Table 2), and the remainder were characterized by more distal disease, but still considered surgically approachable. The operability rate (93%) in this case was much higher than that commonly reported in different series in which it ranges from 10% to 50%32. However, diagnostic advances and growing surgical experience have been important in redefining the distal limits of PEA; at specialized centers, surgery can be successfully performed in selected patients with distal disease. Therefore, published inoperability rates may overestimate actual overall inoperability. As an example, in the CHEST-1 study the central adjudication committee (whose members perform more than 50 PEAs per year) doubled the operability rate relative to that considered by local adjudication committees33.
Despite the small number (n=4), the presence of distally distributed disease was associated with worse hemodynamic outcome and persistent PH after surgery, as described in several studies11,22,34. Perioperative complications were not more frequent in this group. It should be noted that the most recent developments in PEA require more refined definitions of operable CTEPH. More distal obstructions, namely at the subsegmental or even distal segmental level may be considered inoperable at less experienced centers. However, a highly experienced team may consider such cases operable. It is the opinion of high-volume centers that all patients with CTEPH, including those with distal disease, should be evaluated for PEA5.
The aforementioned anatomical distribution of CTEPH provides the rationale for the use of other circulatory optimization strategies. Distal vasculopathy in CTEPH, either thrombotic or ‘reactive’ to hyperflow in areas without thrombotic burden, has been the subject of studies with drugs targeting pulmonary arterial hypertension. Vasodilator therapy is indicated in symptomatic patients with inoperable CTEPH or residual/recurrent PH after PEA. The use of vasodilator drugs in operable CTEPH prior to PEA may improve hemodynamics before the procedure35–37, but does not appear to affect prognosis and hemodynamics post-surgery38. Despite the absence of evidence to support this practice, several centers resort to pharmacological bridge to surgery with the goal of hemodynamic and functional improvement6. The group from the University of California (San Diego, USA) published that about 45% of patients with operable CTEPH were treated with at least one vasodilator drug before referral for PEA8. In the international prospective CTEPH registry, 29% of operated patients received bridge therapy. These patients had worse FC and were more hemodynamically compromised compared to those not medicated. At our center, we medicated most patients (93%; n=25) prior to surgery, based on functional status and hemodynamic outcomes. This practice, currently no longer used, reflects, to some extent, the modus operandi of a center where PEA was not previously considered.
At about four to six months after PEA, we began specific pulmonary vasodilator therapy with at least one drug in 44% (n=12) of patients, a higher rate than that reported by the largest long-term follow-up series of CTEPH patients operated on (21%)4. This decision was based on functional (such as WHO FC and 6MWT) and hemodynamic parameters (mPAP and mRAP), which have been independently associated with the start of therapy.
Recent randomized clinical trials have shown that the soluble guanylate cyclase stimulator riociguate and the endothelin receptor antagonists bosentan and macitentan provided short- to medium-term symptomatic, hemodynamic and functional improvement30,31,39,40. In the CHEST-1 study, mPAP and PVR were reduced by about 10% and 33%, respectively, while 6MWT increased by just over 10% in the riociguate arm and 33% of patients reported a one-point improvement in FC. The safety and efficacy of riociguate appears to extend beyond 2 years40,41, although benefits in long-term mortality have not yet been demonstrated. A very similar positive effect on PVR was demonstrated in the MERIT-1 study with the use of macitentan31. These drugs can be used as monotherapy or in combination with other classes (off-label) — phosphodiesterase inhibitors or prostanoid analogues. However, it must be noted that their effects do not modify the underlying thromboembolic/fibrotic mechanical obstructions and must be taken ad eternum, which entail significant costs and some side effect-related morbidity.
With regard to anticoagulant therapy, vitamin K antagonists are the classically indicated drugs in patients with CTEPH, and there are not many safety, and efficacy data with DOACs, despite their increasing and widespread use. A recent retrospective study by the Papworth group, the largest to date on the use of anticoagulants in CTEPH (794 patients after PEA) found that in 2017-2018, 55% of the population was medicated with DOACs42, a rate comparable to other centers43, including the one in the present study (48%). They found that post-PEA functional, hemodynamic, incidence of bleeding and survival outcomes were independent of the type of anticoagulant. There was, however, a higher incidence of recurrent venous thromboembolism with the use of direct oral anticoagulants (DOACs) (4.62% vs. 0.76%/person-year; p=0.008). Further studies in this area will be needed to define the best anticoagulation strategy in patients with CTEPH.
Patients with CTEPH often require LTOT, as the mechanisms of respiratory failure are multifactorial — low cardiac output state and ventilation-perfusion mismatch. In our series, PEA led to a significant reduction in the number of patients on LTOT — 14 vs seven patients. Additionally, the presence of LTOT pre-PEA was associated with persistent PH. It should be noted that there was a dissociation between hemodynamic improvement and respiratory failure, the former being earlier, as described in other studies44,45. Ishida et al. observed that although 92% of patients returned to WHO functional classes I and II post-PEA, 37% would still be dependent on LTOT25. Potential underlying mechanisms include the presence of post-surgery lung restriction and the greater latency in restoring ventilation/perfusion in previously obstructed areas due to reperfusion edema and the effect of ‘coronary steal’ from obstructed areas and with higher PVR for unobstructed areas.
The incidence, prevalence, and epidemiology of CTEPH in Portugal are not yet widely known. These data are difficult to obtain because it is an underdiagnosed46 disease, which reflects the non-specific characteristics of the signs and symptoms, limited recognition of the disease and inefficient application of diagnostic tests23,47,48. Registry data suggest a prevalence in the general population of 3-30/million49. In the first50 Portuguese multicenter prospective registry of patients with PH and CTEPH, the cases of CTEPH represented 41.8% of the population.
In this study, the median times from symptom onset to diagnosis and from diagnosis to surgery were 26 (14-53) and 16 (8-48) months, respectively. To provide the best approach for patients with CTEPH, it is essential to increase the medical profession’s awareness of the disease, thus avoiding diagnostic and therapeutic delays.
Classically, CTEPH is considered a complication of acute PE, with a described prevalence of between 2% and 4% after the event14,44,51. However, not all patients with CTEPH have a history of acute PEA. In our series, 16 (59%) patients reported a history of acute PE. A large prospective international CTEPH registry reported that 75%-80% of included patients had a history of acute PE23,47. However, this frequency is probably overestimated, since the diagnosis of acute PE has not been properly documented in a significant number of patients. Additionally, it is hypothesized that several cases described as acute PE could be early manifestations of CTEPH.
Concluding remarksThe main limitation of this study was the difficulty in compiling clinical data, since it took place at two centers located in different countries. However, this is the only study to date that presents an international partnership in the multidisciplinary treatment of this disease.
A Portuguese center for the surgical treatment of CTEPH has recently been appointed by the Portuguese health authority. However, there are still no published objective data on the post-surgery hemodynamic results, the essential gold standard of the efficacy and quality of surgery in CTEPH, to allow for important comparison to be made by our scientific community. This will allow clinicians who follow these patients to decide which is the best solution in each case.
National experience of cooperation with another country shows the safety of the protocol paving the way for the Portuguese center — the same may be applied in Portugal, now as a surgical center, enabling referral from the Portuguese islands and other countries, in particular, the Portuguese-speaking African countries, as is already the tradition in Portugal.
ConclusionThe literature review and current experience demonstrate that PEA performed at a highly differentiated and experienced surgical center is a safe procedure with a very favorable mid-term impact on functional, hemodynamic, and right ventricular function parameters in patients with operable CTEPH.
The first stage of surgical success begins with early diagnosis of CTEPH, targeted therapy and patient stabilization to allow for surgery to take place. The role of a differentiated medical center is again critical for morbidity and mortality outcomes.
This study shows that it is possible for clinical centers specialized in the treatment of PH without PEA differentiation, to refer patients safely and effectively to an international surgical center where air transportation is necessary.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Plácido R, Guimarães T, Jenkins D, Cortez-Dias N, Pereira SC, Campos P, et al. Hipertensão pulmonar tromboembólica crónica: experiência inicial de doentes submetidos a tromboendarterectomia pulmonary. Rev Port Cardiol. 2021;40:741–752.