Chagas disease is among the neglected tropical diseases recognized by the World Health Organization that have received insufficient attention from governments and health agencies.
Chagas disease is endemic in 21 Latin America regions. Due to globalization and increased migration, it has crossed borders and reached other regions including North America and Europe. The clinical presentation of the disease is highly variable, from general symptoms to severe cardiac involvement that can culminate in heart failure. Chagas heart disease is multifactorial, and can include dilated cardiomyopathy, thromboembolic phenomena, and arrhythmias that may lead to sudden death. Diagnosis is by methods such as enzyme-linked immunosorbent assay (ELISA) and the degree of cardiac involvement should be investigated with complementary exams including ECG, chest radiography and electrophysiological study. There have been insufficient studies on which to base specific treatment for heart failure due to Chagas disease. Treatment should therefore be derived from guidelines for heart failure that are not specific for this disease. Heart transplantation is a viable option with satisfactory success rates that has improved survival.
A doença de Chagas é uma das reconhecidas pela Organização Mundial de Saúde (OMS) entre um grupo de doenças que receberam atenção insuficiente de governos e órgãos de saúde.
A doença de Chagas é endémica em 21 regiões da América Latina. Devido à globalização e ao aumento da migração, a doença de Chagas cruzou fronteiras e atingiu outras regiões como a América do Norte e a Europa. A apresentação clínica da doença pode ser muito variada, pois gera desde sintomas gerais até comprometimento cardíaco grave, capaz de culminar em insuficiência cardíaca. A cardiopatia é multifatorial e, dessa forma, pode expressar-se em miocardiopatia dilatada, fenómenos tromboembólicos e arritmias que podem levar à morte súbita. O diagnóstico deve ser feito utilizando métodos como o estudo imunoenzimático (ELISA) e a investigação do grau de acometimento cardíaco deve ser realizada com outros exames complementares, como ECG, radiografia do tórax, estudo eletrofisiológico, entre outros. Os estudos científicos são insuficientes para propor um tratamento específico para insuficiência cardíaca por doença de Chagas. Portanto, o tratamento deve ser extrapolado das diretrizes para insuficiência cardíaca que não são específicas para essa doença. Atualmente, sabe-se que o transplante cardíaco é uma opção viável que melhora a sobrevida e apresenta taxas de sucesso satisfatória.
Chagas disease was first described by the Brazilian physician Carlos Chagas in 1909.1–3 He identified the etiologic agent, the parasite Trypanosoma cruzi, as well as elucidating its life cycle.4 It is a neglected tropical disease which is transmitted via the feces of a triatomine-infected vector through a bite wound or mucous membrane.1,5 Oral and vertical transmission, blood transfusion and organ transplantation are other possible routes of dissemination.6
Chagas disease used to be endemic, restricted to Latin America. However, increased immigration has transformed this local problem into a global public health issue. It affects between eight and 12 million adults around the world, including in Europe, North America, Japan and Australia.1,7,8
Chronic Chagas cardiomyopathy occurs in 20-40% of infected individuals, and is its most important consequence, as it is potentially lethal. This condition has various manifestations, including arrhythmias, heart failure, stroke, heart block, thromboembolism and sudden death.9 Megaesophagus, megacolon and autonomic nervous system dysfunction are common extracardiac complications.1
In this article, we review the epidemiology, pathophysiology, clinical manifestations, treatment and prognosis of Chagas disease, focusing on its cardiac consequences. A thorough understanding of Chagas disease and its complications is crucial to the correct management of this disease.
EpidemiologyChagas disease is endemic in 21 Latin America regions. Traditionally, the disease was transmitted through the vector, which lives in the mud walls of rural houses in developing countries. However, patterns of migration and new forms of dissemination have led to the current geographic distribution of the disease.10 Its prevalence has decreased in recent years due to blood transfusion screening and vector control.11
Currently, the area with the highest incidence of Chagas disease is the Bolivian Chaco, where the infection rate is 4% per year and where it is the most common cause of non-ischemic cardiomyopathy in the country, affecting 7% of adults.7,12 In Brazil, the distribution of the disease is uneven, in line with the country's continental proportions.13 Between 2008 and 2017, 2086 new cases of acute Chagas disease were reported in Brazil, 95.3% of which were in the north: the state of Pará had the highest number of cases in that period (1739), while the Northeast reported 56 new patients. The areas with the lowest incidence were the Federal District of Brasilia and the states of Piauí, Espírito Santo and Rio de Janeiro, with only one case each.13
Vector control programs begun during the twentieth century brought benefits to Latin America and by the end of the century had succeeded in eradicating vector transmission in Uruguay, Chile and some regions of Brazil. As other forms of disease spread are less important in these areas, the risk of transmission of Chagas disease is close to zero at these sites.14–16 However, due to globalization and increased migration, Chagas disease has crossed the borders of endemic regions and reached North America, Europe, Australia and Japan.17 The prevalence of Chagas disease in Latin Americans living in Europe is around 4.2%.18 In the US, it is estimated that more than 100 000 individuals are affected by the disease.19
In a study conducted in New York City, analysis of a sample of patients from Latin America with dilated cardiomyopathy concluded that Chagas disease was the cause of cardiac involvement in up to 13% of cases.20 However, the disease is considered to be underdiagnosed, with an estimated 95% of cases being undiagnosed.21 Thus, data on the disease and its cardiovascular repercussions are imprecise.
Etiology and pathophysiologyThe etiologic agent of Chagas disease is T. cruzi, which occurs in two forms when it infects humans: trypomastigotes and amastigotes. The agent circulates via blood-sucking triatomine vectors and presents a genetic variety that determines its pathogenicity and response to therapy. Currently, T. cruzi is divided into six discrete typing units (TcI-TcVI).22
The most prevalent form of transmission of the disease is by vector, mainly in endemic areas. The cycle of the etiological agent is divided into the vector stage and human stage. Initially, a triatomine bug bites a human and simultaneously passes feces infected with the metacyclic trypomastigote form of the parasite, thus beginning the human stage. The parasites then penetrate the site of the bite or a mucous membrane and invade several cells near the penetration site, where they transform into amastigotes. The latter multiply by binary division, transform into trypomastigotes and burst out of the host cell, entering the blood circulation. The parasite can thereby infect new cells, where it turns into the amastigote form, restarting the cycle or being ingested by a new vector feeding on host blood.23
If it is ingested by the vector, the vector stage of the cycle begins. The trypomastigote form becomes an epimastigote in the midgut of the vector and begins to replicate. In the hindgut, epimastigotes differentiate into metacyclic trypomastigotes that are then eliminated in the feces of the vector, giving rise to a new cycle of infection.23
The Amazon Basin region, which has the best domestic vector control, has seen a significant increase in oral transmission by foods contaminated with T. cruzi. In Brazil between 2008 and 2017, 69.5% of new cases of the acute form of Chagas disease were attributed to oral transmission of the disease.13 Chile and Uruguay managed to halt vector transmission in the 2000s.2
Other forms of disease transmission are found in both endemic and non-endemic countries. Blood dissemination has an estimated transmission rate of 10-25%.24,25 Infection by solid organ transplantation from infected donors varies according to what has been transplanted. The estimated rate for kidney transplantation ranges from 0-13% while the rate for infected heart recipients may reach 100%.26–28
Vertical transmission is also reported, with an estimated rate of 3.9-5.6%.29 There are important factors that influence the transmission potential of this mechanism. High maternal parasitemia increases the probability of transmission, depending on which discrete typing units are involved. A study conducted in Brazil suggested that TcII has a lower transmission rate than TcV.30 Ingestion of contaminated food and laboratory accidents have been reported, but are less frequent forms of transmission.31,32
The disease presents an initial acute phase that begins 1-2 weeks after infection and lasts for up to 4-8 weeks.33 At this stage, there is extensive parasitism and infection, particularly of certain tissues such as esophageal, colon and heart muscle and the central nervous system.34,35 The parasite infects these target tissues by transmigration through the vascular barrier with the aid of bradykinin and the chemokine CCL2.36 The process may elicit an immune response mediated by activation of CD4+ and CD8+ T cells and B cells.37 As a result, myocarditis may develop with mononuclear and polymorphonuclear infiltrates, interstitial edema, vasculitis, and myocyte necrosis.34,35
After this stage, the chronic phase begins, at which point the heart may become involved. The pathogenesis of Chagas cardiomyopathy is the result of a range of aggressive mechanisms that culminate in cardiac injury, the main ones of which are parasite persistence, immunological mechanisms, autonomic nervous system derangement and microcirculatory disturbances.38
Parasite persistence appears to be the most important mechanism in the genesis of Chagas cardiomyopathy. The parasite has mechanisms to perpetuate the infection, such as inhibition of cardiomyocyte caspase activity mediated by nuclear factor kappa B and the Bcl-2 protein.39 During the chronic phase, the amastigote coexists with the host cell, generating constant low-grade inflammation leading to chronic myocarditis. Thus, parasite persistence leads to fibrosis and cardiac remodeling.40,41
During the chronic phase of the disease, there is an exaggerated type 1 T helper (Th1) immune response with release of interferon gamma and tumor necrosis factor alpha, decreased release of interleukin (IL)-10, and suppression of Th2 response-related cytokines such as IL-4.42,43 In addition, polymorphisms in inflammatory genes (BAT1, membrane cofactor protein-1, lymphotoxin alpha) have been reported in relation to the genesis of chronic Chagas cardiomyopathy.44,45
Neurogenic disturbances are involved in at least three different aspects of the development of the disease.38 Early parasympathetic impairment is present in patients with malignant arrhythmias and sudden death.46 In early stages of the disease, dyssynergic areas are found in both ventricles. Finally, it is thought that autonomic derangement may trigger microcirculatory vasospasm.38
Chagas cardiomyopathy results in changes in the microvascular circulation. There are reports of chronic perivascular inflammation, capillary basement membrane thickening, increases in prothrombotic components, factors leading to aneurysm formation, alterations in vasoconstriction and vasodilation leading to ischemia, necrosis, and reparative fibrosis.38
Microscopically, mononuclear cell infiltration is observed, composed of varying numbers of lymphocytes, plasma cells, histiocytes, and eosinophils. Granulomas and multinucleated giant cells may also be found. Intracytoplasmic amastigote forms are seen less frequently.47
In autopsies, varying degrees of biventricular dilatation, hypertrophy and fibrosis of the myocardium and epicardium are found. Other reported alterations include left ventricular aneurysms and mural thrombi that are not exclusive to Chagas disease.47 Epicardial plaques (known as ‘milk spots’) may also be observed; these are rarer in the general population and are more specific to Chagas cardiomyopathy.47,48
Clinical presentationThe clinical presentation of Chagas disease is divided into three phases: acute, indeterminate and chronic. After an incubation period of 1-2 weeks,49 the acute phase of the disease begins, which lasts from six to eight weeks.50 Although most patients show no early signs, non-specific symptoms may occur in the acute phase, including fever, malaise, muscle pain, hepatosplenomegaly, anorexia, vomiting and diarrhea. In a few cases, signs at the infection site may be present: Romaña's sign (painless prolonged edema of the eyelid) and chagoma (elsewhere on the skin).50 Fewer than 1% of cases present with meningoencephalitis or myocarditis in the acute phase due to the severe form of the disease.51 Cardiac complications in the initial phase are more frequently associated with oral transmission than vector-borne infection, due to better control of T. cruzi inside homes.52
Usually, the acute phase of the disease resolves spontaneously even without therapeutic intervention and the patient becomes chronically infected. Thus, most patients develop the indeterminate form of the disease, which is characterized by absence of symptoms or signs of cardiac or digestive tract involvement and no alterations on the electrocardiogram (ECG) or chest X-ray, but with seropositivity for T. cruzi. This form of the disease presents an excellent prognosis.53
Decades after the acute and indeterminate phases, less than half of patients develop the cardiac and/or gastrointestinal involvement that characterizes the chronic stage of Chagas disease. Gastrointestinal manifestations occur in 15-20% of patients, including dilatation of the gastrointestinal tract, mainly the esophagus and colon (rectum and sigmoid).54 This dilatation is due to loss of muscle tone as a consequence of chronic inflammation of the enteric plexus and degeneration of intestinal neurons.55 The spectrum of esophageal disease presentation is varied, ranging from mild asymptomatic achalasia to severe megaesophagus with regurgitation, aspiration, cough, dysphagia, odynophagia and weight loss.56 Colon disease manifests as megacolon with chronic constipation leading to fecalomas, volvulus and even ischemia of the region.57
Chronic Chagas cardiomyopathy is estimated to affect 20-30% of infected patients.49 Cardiac symptoms arise with the progressive destruction of cardiomyocytes, accumulation of interstitial collagen, and damage to the cardiac conduction system.38 Chagas heart disease occurs in a segmental pattern and may present a wide range of alterations depending on the cardiac site affected. These changes frequently include dilated cardiomyopathy, thromboembolic phenomena, and arrhythmias that may lead to sudden death.
Early cardiac changes may include multiform premature ventricular contractions, right bundle branch block or left anterior fascicular block.58,59 Its highly arrhythmogenic and progressive nature may lead to persistent changes in the conduction system, such as severe bradycardia due to sinus node dysfunction, atrial fibrillation, atrial flutter, atrioventricular (AV) block, premature ventricular contractions, and sustained and non-sustained ventricular tachycardia.5,49,58,59 At this stage of the disease, the patient may have preserved ventricular function. Pulmonary and systemic thromboembolic phenomena are reported, as well as apical aneurysm formation.60,61
Infected patients may develop biventricular dilated cardiomyopathy and congestive heart failure.60 A Brazilian study of infected blood donors showed a progression to cardiomyopathy of 1.85% per year.62 At the present time, this form of transmission is no longer found because of screening of blood donors. It has also been observed that regions with effective vector control presented a lower prevalence of severe Chagas cardiomyopathy because patients did not present an inflammatory response due to repeated contact with the antigen.63
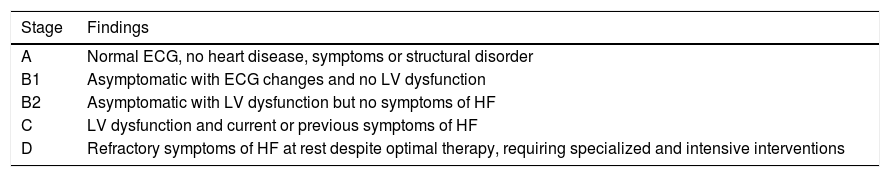
Cardiovascular involvement in the chronic phase of Chagas disease can be classified according to the presence of ventricular dysfunction and heart failure symptoms according to Latin American guidelines for the diagnosis and treatment of Chagas’ heart disease (Table 1).50
Clinical staging of chronic heart failure according to findings of follow-up exams.
| Stage | Findings |
|---|---|
| A | Normal ECG, no heart disease, symptoms or structural disorder |
| B1 | Asymptomatic with ECG changes and no LV dysfunction |
| B2 | Asymptomatic with LV dysfunction but no symptoms of HF |
| C | LV dysfunction and current or previous symptoms of HF |
| D | Refractory symptoms of HF at rest despite optimal therapy, requiring specialized and intensive interventions |
ECG: electrocardiographic; HF: heart failure; LV: left ventricular.
Patients in stage A have no cardiac alterations and are thus in the indeterminate phase of the disease. In stage B1, individuals are asymptomatic but present alterations on the ECG or on echocardiography. Stage B2 indicates asymptomatic patients with ventricular dysfunction but no heart failure, while stage C indicates the presence of ventricular dysfunction and current or previous symptoms of heart failure due to Chagas cardiomyopathy. In stage D refractory heart failure symptoms are present at rest even with optimal therapy. At this stage patients need intensive interventions.
Heart failure syndrome begins with exertion-related dyspnea and fatigue. As the condition progresses, the symptoms and signs of pulmonary and systemic congestion become more evident. Subsequently, paroxysmal nocturnal dyspnea, orthopnea, jugular venous engorgement, hepatomegaly, worsening lower limb edema and anasarca appear. Cardiovascular signs may include displacement of the apex beat, muffled cardiac sounds, third heart sound, or systolic regurgitation murmur due to dilation of the annuli of the AV valves. Reduced systemic blood pressure associated with thready pulse may also be present. Crackles on pulmonary auscultation demonstrate pulmonary congestion.
Arrhythmic manifestations may arise in conjunction with heart failure syndrome or separately. Clinical examination may reveal irregular heart rhythm, bradycardia or tachycardia, or splitting of the second heart sound. In complete AV block, cannon A waves in the jugular venous pulse may be observed at the base of the neck. The most common intraventricular conduction abnormality found in Chagas cardiomyopathy is right bundle branch block, which can affect up to 28.8% of patients.64-66 When associated with anterior fascicular block, the condition becomes more characteristic of the disease and requires further investigation. Left bundle branch block is less frequent and when present is indicative of worse prognosis.64-66 The most common atrial tachycardia is atrial fibrillation, which affects up to 5.4% of patients.64 It is strongly associated with stroke risk and is an important predictor of mortality.66 Monomorphic or polymorphic premature ventricular complexes are commonly found ventricular tachyarrhythmias, affecting up to 66% of patients.67 Tachyarrhythmias originating in the ventricles are strongly associated with the occurrence of sudden death. Ventricular tachyarrhythmias may be related to cardiac areas with perfusion deficits and hypokinesia, with the inferolateral region of the left ventricle being the most common focus.68–70
Stroke and systemic embolism are possible manifestations in the chronic phase of Chagas disease. The origin of the thrombi is usually inside the cardiac chambers, related to loss of function and atrial arrhythmias.71,72 Atrial fibrillation, left ventricular systolic dysfunction, apical aneurysm and left ventricular thrombus are reported as risk factors for stroke.71,73 However, non-embolic causes of stroke are also found, including cryptogenic (25.5%), small vessel disease (9.5%), and large vessel atherosclerosis (8.5%). Presentation is similar to those without Chagas disease, such as motor or sensory deficit, dysarthria, aphasia and visual changes.74
Some studies reveal cognitive alterations and vascular dementia in endemic regions, even in patients without cardiac alterations.75 Cerebral atrophy has been found in patients in the chronic phase of the disease with no relation to brain infarcts.76,77 However, studies have not found the parasite in brain tissue77 and atrophy is believed to be a manifestation of the multifactorial nature of the disease and its systemic involvement.76 Cardiac arrhythmias and congestive heart failure leading to chronic hypoxemia, reduced Purkinje cell numbers, spinocerebellar tract and dorsal column demyelination, extensive cell loss in the substantia nigra and locus coeruleus, and a basal ganglia lacunar state are reported as alterations that may lead to neurological manifestations.78
DiagnosisIn the acute phase, the most sensitive diagnostic method is identification of T. cruzi genetic material by polymerase chain reaction.33 Other diagnostic forms used include xenodiagnosis, blood culture, direct visualization of the parasite in peripheral blood, and biopsy of infected organs. At this early stage of the disease, electrocardiographic changes may already be detected, mainly in patients with myocarditis. The examination may reveal sinus tachycardia, low QRS voltage, prolonged PR and/or QT interval, ventricular repolarization abnormalities, atrial fibrillation and ventricular arrhythmias.50
The indeterminate form is characterized by serological or parasitological evidence of the infection, but absence of signs or symptoms or changes on the ECG or chest X-ray. There may be slight changes in more sensitive exams such as echocardiography, cardiac magnetic resonance imaging (MRI), Holter monitoring, myocardial scintigraphy or endomyocardial biopsy. However, studies do not correlate these findings with the prognosis of the disease.50
In the chronic phase, parasitemia is low and therefore the most effective diagnostic methods involve identification of antibodies against the etiologic agent. The main techniques used are indirect immunofluorescence, enzyme-linked immunosorbent assay (ELISA) and indirect hemagglutination.
The earliest changes indicative of Chagas cardiomyopathy usually appear on the ECG. Right bundle branch disorders and left anterior hemiblock are highly characteristic findings of this stage of cardiac involvement. The other above-mentioned arrhythmias can also be detected on the ECG. Polymorphic ventricular extrasystoles, atrial fibrillation and atrial flutter are associated with more advanced ventricular dysfunction. Ventricular arrhythmias may occur in patients without ventricular dysfunction, but tend to be related to more advanced stages and worse prognosis. Patients who present left ventricular dysfunction and/or syncope are indicated for Holter continuous electrocardiographic monitoring to detect bradyarrhythmias or tachyarrhythmias that are not observed on a routine ECG.50
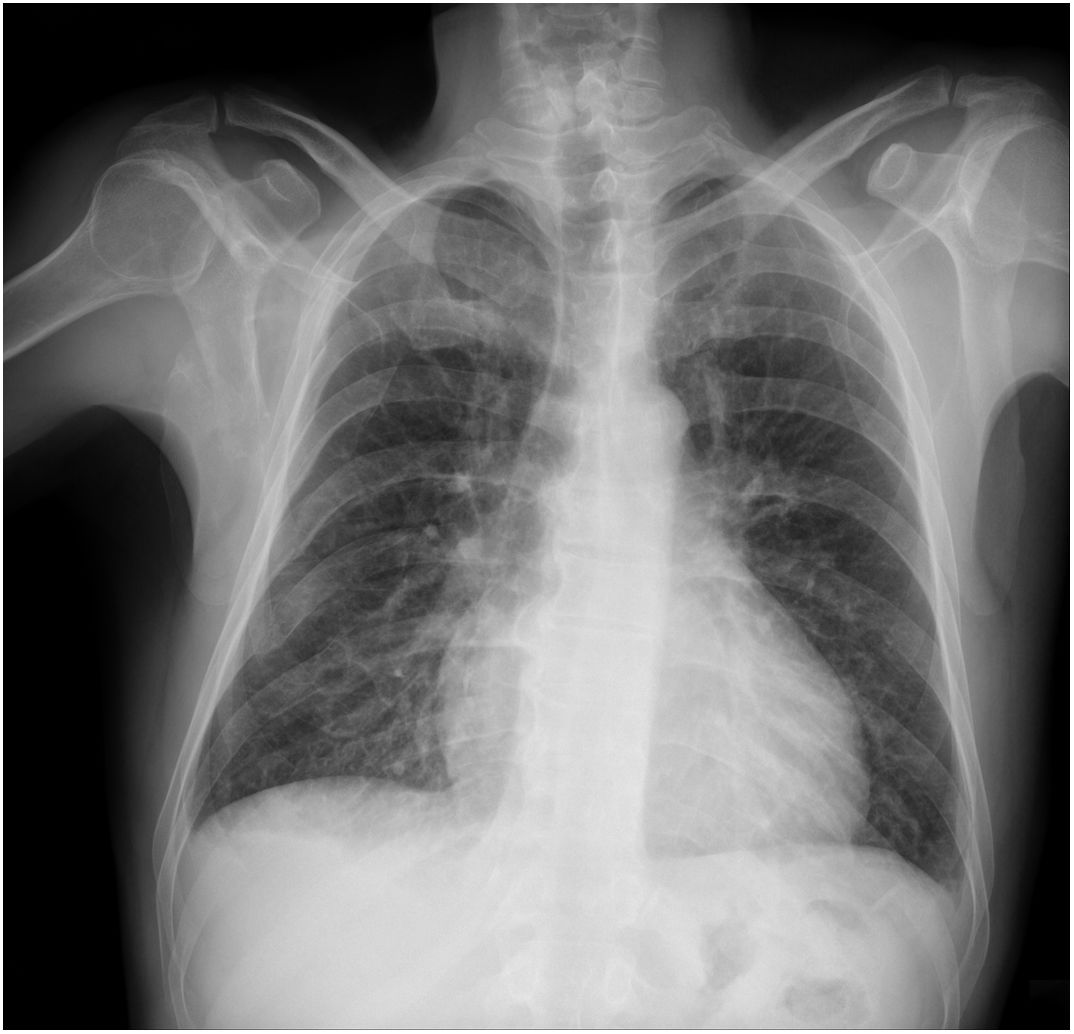
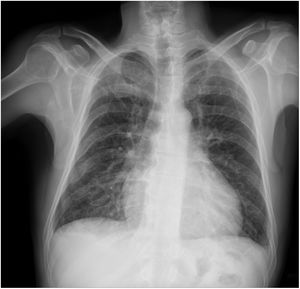
Chest radiography may be useful for assessing the disease in more advanced stages (Figure 1). Cardiomegaly with enlargement of the right and left heart chambers may be observed. However, pulmonary congestion is usually mild or non-existent.50
Echocardiography is indicated for all patients presenting with Chagas disease, in whom it can assess systolic and diastolic synchronicity and the presence of intracavitary thrombi and apical aneurysms. In the indeterminate phase, some patients may present left ventricular segmental wall motion abnormalities even if a previous ECG was normal. In more advanced stages, the examination may reveal dilatation of the cardiac chambers associated with global hypokinesia. Dilatation of valve annuli secondary to cardiomegaly leads to mitral and/or tricuspid regurgitation. Ventricular aneurysms are also a common finding in this phase and are associated with up to 67% of cases associated with high thromboembolic risk.50 Echocardiography is not routinely indicated for patients with Chagas cardiomyopathy. Eligible patients are those with new electrocardiographic changes, worsening functional class or a new cardiovascular event.67
Radioisotope ventriculography is an alternative to echocardiography that provides the most accurate assessment of ventricular function for patients with Chagas disease.79 This exam does not suffer from geometric interference from the heart when calculating left ventricular ejection fraction (LVEF) and is able to assess right and left ventricular dysfunction simultaneously and the presence of ventricular dyssynchrony.80,81
Stress testing is useful for the screening and prognosis of stress-induced arrhythmias. Cardiopulmonary exercise testing and myocardial perfusion scintigraphy are viable options to assess coronary perfusion; scintigraphy shows segmental perfusion defects in about 30% of patients with anginal pain and normal coronary angiography.50
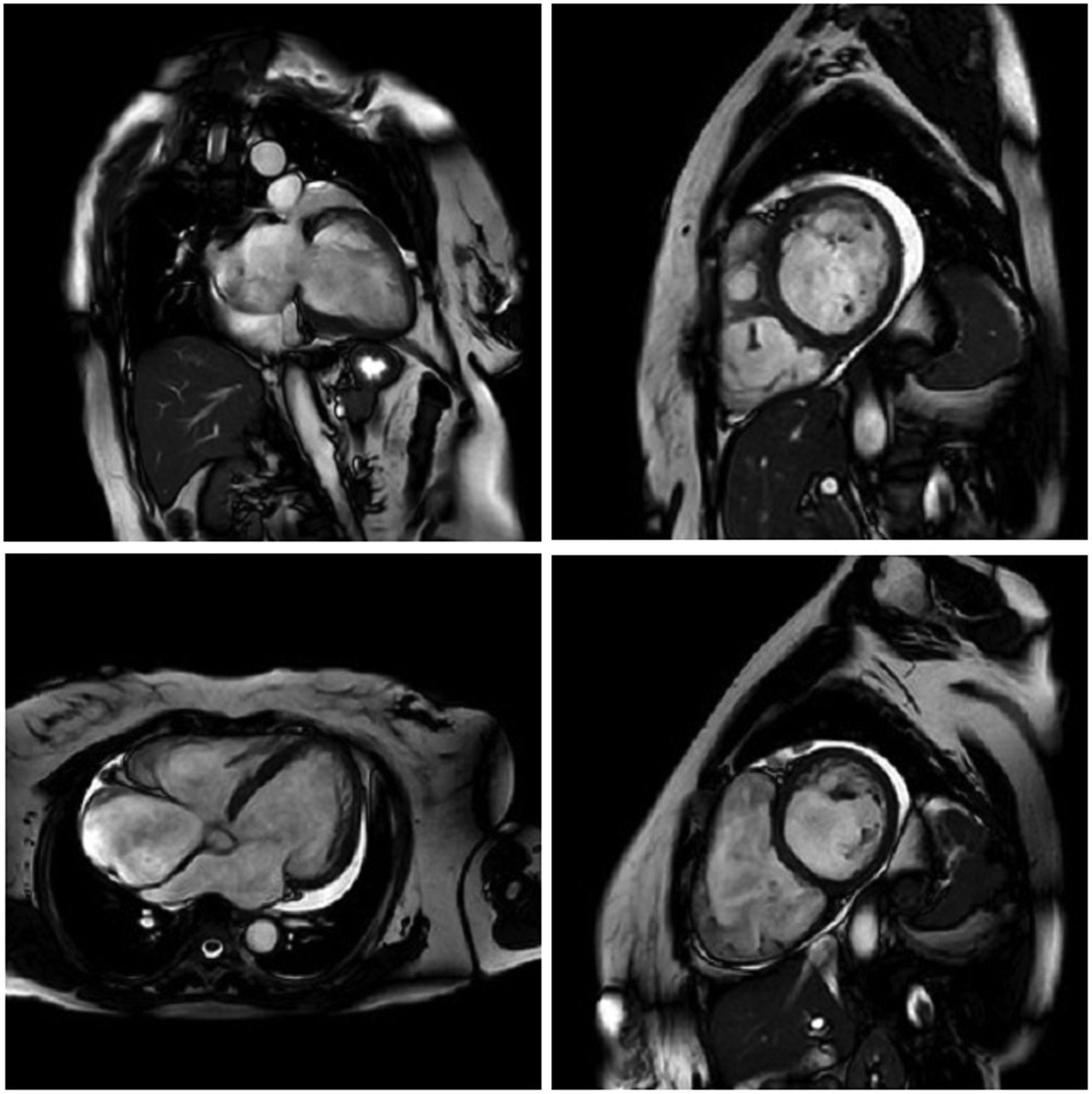
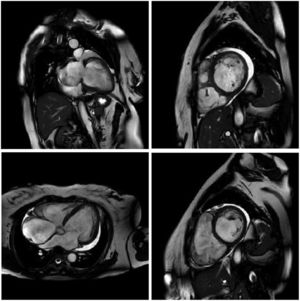
MRI is a high-quality and well-established test for assessing cardiac anatomy and function (Figure 2). It is able to determine cardiac chamber volume and degree of contractility, as well as to diagnose thrombus if present. The most effective analysis of cardiac tissue is by infusion of gadolinium, which can identify and quantify areas of the heart with fibrosis.82,83
Cardiac magnetic resonance imaging showing congestive heart failure/cardiomyopathy of probable inflammatory etiology (Chagas myocarditis), with significant dysfunction and increased biventricular diameters, significantly dilated right atrium and tricuspid regurgitation, dilated left atrium and mitral insufficiency, and pericardial effusion.
Some patients develop malignant arrhythmias without global ventricular systolic dysfunction but with myocardial fibrosis that can be detected by MRI.84,85
Electrophysiological study (EPS) can be used to assess sinus node function and AV conduction in patients with signs and/or symptoms for which there is no pathophysiological explanation documented by non-invasive tests. The main indications for EPS are syncope, resuscitated sudden death and tachyarrhythmias which can potentially be controlled by ablative therapy.50
Cardiac catheterization is indicated for patients with Chagas cardiomyopathy who present with angina symptoms, electrocardiographic alterations compatible with myocardial ischemia, and/or segmental left ventricular wall motion abnormalities on echocardiography. The ECG may reveal pathological Q waves and ST-T segment changes. In the vast majority of cases, the examination reveals no significant changes in the epicardial coronary arteries.67,84 The procedure can measure pulmonary vascular resistance and is thus indicated for patients eligible for heart transplantation to assess the feasibility of the procedure.67
TreatmentIn the acute phase, antiparasitic treatment is recommended for all patients. Regardless of the mechanism of infection, benznidazole and nifurtimox are the drugs of choice and can reduce the duration of illness and its symptoms. Parasitic cure after treatment is achieved in 60-90% of patients undergoing treatment.86–88
Antiparasitic treatment of the indeterminate and chronic forms remains controversial. According to the BENEFIT trial, a randomized study with more than 2800 patients that assessed the efficacy of benznidazole in Chagas disease, there was no benefit in preventing progression of cardiac involvement in patients with established Chagas cardiomyopathy. Thus, patients with established heart disease should not receive routine treatment with benznidazole.89 However, there is consensus that trypanocidal therapy should be provided to female patients of reproductive age in order to prevent vertical transmission and in cases of reactivation by immunosuppression.87
Although there are no studies that prove the effectiveness of pharmacological treatment for Chagas heart disease, the treatment of heart failure is based on conventional treatment for dilated cardiomyopathy of other etiologies. Therapy for systolic heart failure consists of a combination of beta-blockers and angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs). For patients in New York Heart Association (NYHA) functional class II to IV and with LVEF ≤35%, an aldosterone receptor antagonist may be added. For patients with persistent symptoms who tolerate ACEIs or ARBs, these may be replaced by sacubitril/valsartan. In view of the risk of bradyarrhythmia in this condition, it is important to monitor the patient's heart rate, since beta-blockers lower heart rate.50,67,90
Heart transplantation is an alternative for patients with Chagas cardiomyopathy. Survival at one year (71%) and 10 years (46%) after transplantation is better than in patients who undergo the procedure for other reasons.91 A possible complication of this treatment is reactivation of the disease secondary to post-transplantation immunosuppression; this should be treated with benznidazole.92 Some studies show that a reduced immunosuppressive regimen does not increase allograft rejection in patients and is associated with lower rates of reactivation of Chagas disease.93,94 Recently, the first heart transplantation in a patient with Chagas cardiomyopathy in Portugal was performed. After one year of follow-up, the patient improved from NYHA class IV to class I, with no sign of rejection or disease reactivation.95
Patients with symptomatic sinus node dysfunction or advanced AV block are eligible for pacemaker implantation. Precise indications do not usually differ from those for other underlying conditions.50 Left bundle branch block associated with heart failure can be treated with resynchronization therapy. For this, the patient should preferably present LVEF ≤35%, QRS >130 ms and sinus rhythm. However, there is scarce evidence to support this procedure for patients with Chagas cardiomyopathy and right bundle branch block.67
Ventricular arrhythmias are an important risk factor for sudden death in patients with Chagas cardiomyopathy. However, there is no fixed protocol for the management of these complications. Patients with preserved ventricular function have no indication for treatment. Although there is no evidence that it prevents sudden death, patients with both ventricular arrhythmias and heart failure are eligible for treatment with amiodarone associated with drugs for ventricular dysfunction with reduced LVEF. Amiodarone improves symptoms and reduces the density of ventricular arrhythmias.67
An implantable cardioverter-defibrillator (ICD) is indicated for patients with arrhythmias with high mortality such as sustained ventricular tachycardia or ventricular fibrillation, or who have had an episode of resuscitated sudden death. The use of this device led to a 72% reduction in mortality compared to amiodarone alone.96 Patients with preserved ventricular function may be more difficult to identify, making ICD indication more difficult. After ICD placement, amiodarone plus a beta-blocker should be considered in order to avoid the deleterious effects of frequent shocks.97 Patients with well-tolerated sustained ventricular tachycardia and preserved ventricular function may undergo ablative therapy associated with amiodarone to avoid repeated shocks. However, the safest option is an ICD.50
PrognosisThe prognosis of Chagas disease is highly variable, as the condition can affect various systems, and in the case of cardiomyopathy its pathophysiology is complex, leading to a wide range of disease phenotypes. The main cause of mortality in the chronic phase is sudden death, accounting for 55-65% of deaths.98
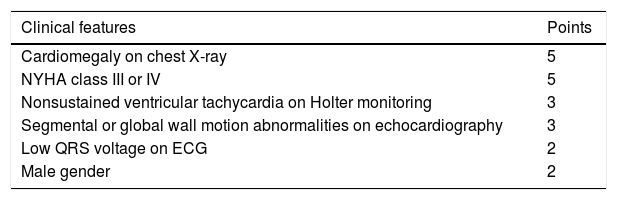
The Rassi score (Table 2) is used to stratify mortality risk in patients in the chronic phase and with cardiac involvement.99 The variables assessed in the score are gender, low QRS voltage on ECG, nonsustained ventricular tachycardia, global or segmental left ventricular wall motion abnormalities, cardiomegaly on chest X-ray and heart failure (NYHA class III or IV). The score estimates 10-year mortality risk, classifying patients as high risk (12-20 points), intermediate risk (7-11 points) or low risk (0-6 points). In the development and validation cohorts on which the score was based, the 10-year mortality rates for high, intermediate and low risk patients were 84-85%, 37-44% and 9-10%, respectively.99 The presence of nonsustained ventricular tachycardia is associated with a 2.15-fold increased risk of mortality, and the combination of this arrhythmia with left ventricular systolic dysfunction is associated with a 15.1-fold increased risk for subsequent death.100
Rassi score for mortality risk stratification in Chagas disease.
| Clinical features | Points |
|---|---|
| Cardiomegaly on chest X-ray | 5 |
| NYHA class III or IV | 5 |
| Nonsustained ventricular tachycardia on Holter monitoring | 3 |
| Segmental or global wall motion abnormalities on echocardiography | 3 |
| Low QRS voltage on ECG | 2 |
| Male gender | 2 |
ECG: electrocardiogram; NYHA: New York Heart Association.
Chagas disease is a neglected illness that has crossed the borders of endemic regions and reached North America, Europe, Australia and Japan. Nowadays, oral transmission is the main mechanism for perpetuating the disease.
The systemic involvement of the disease affects various organs including the esophagus, colon, heart and central nervous system. Cardiac changes in Chagas disease include dilated cardiomyopathy, thromboembolic phenomena, and arrhythmias that may lead to sudden death. Thus, investigation of this stage of the disease should be aimed at these possible complications.
There is no specific treatment for Chagas heart disease. Clinical management of heart failure is based on conventional treatment for dilated cardiomyopathy. Heart transplantation is an alternative for patients with Chagas cardiomyopathy and has satisfactory success rates. The prognosis of the disease is variable and the Rassi score is used to stratify the mortality risk of these patients.
Conflicts of interestThe authors have no conflicts of interest to declare.