Defibrillation tests during implantation of a cardioverter-defibrillator (ICD) assess the device's effectiveness in detection and termination of ventricular fibrillation (VF). An appropriate safety margin is considered to be 10J above the minimum value tested, but the utility of such tests in the context of primary prevention has recently been called into question by various authors.
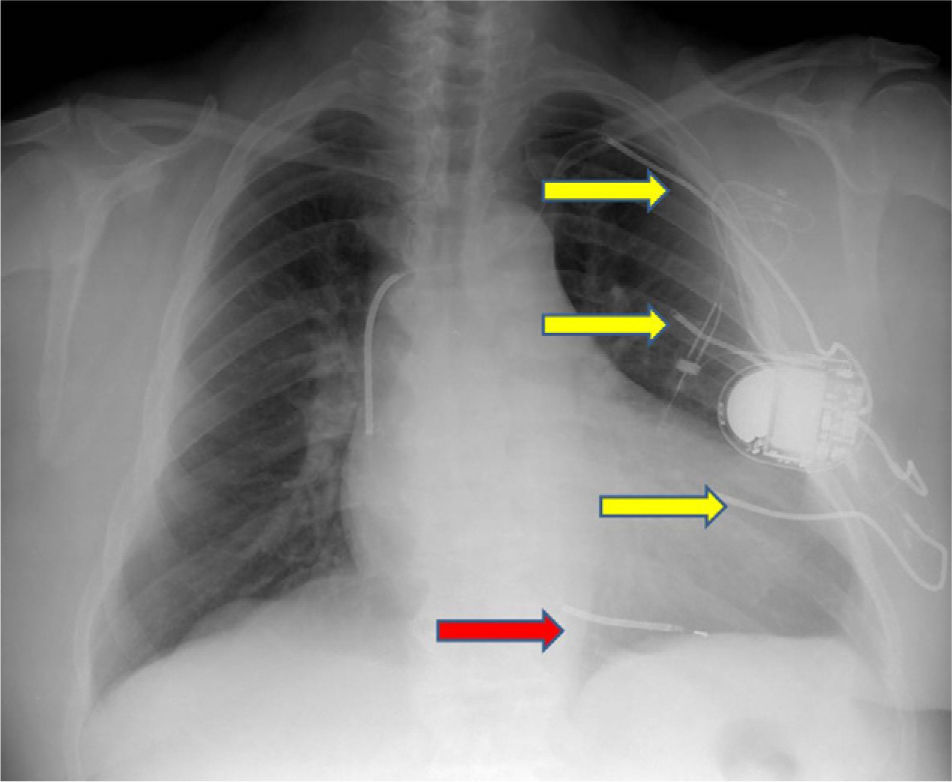
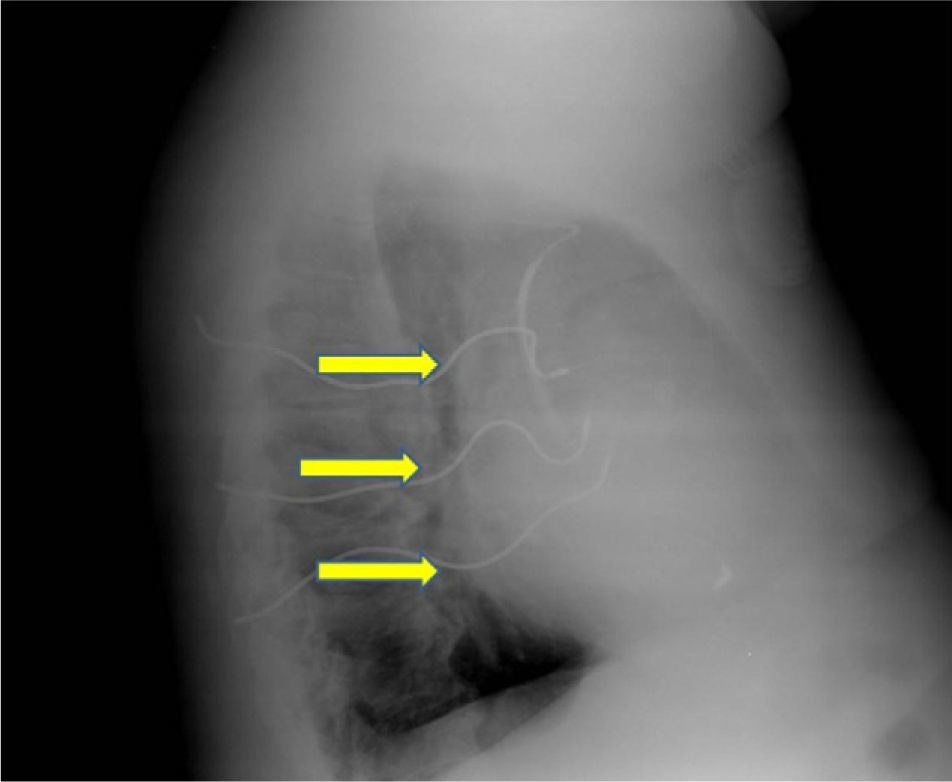
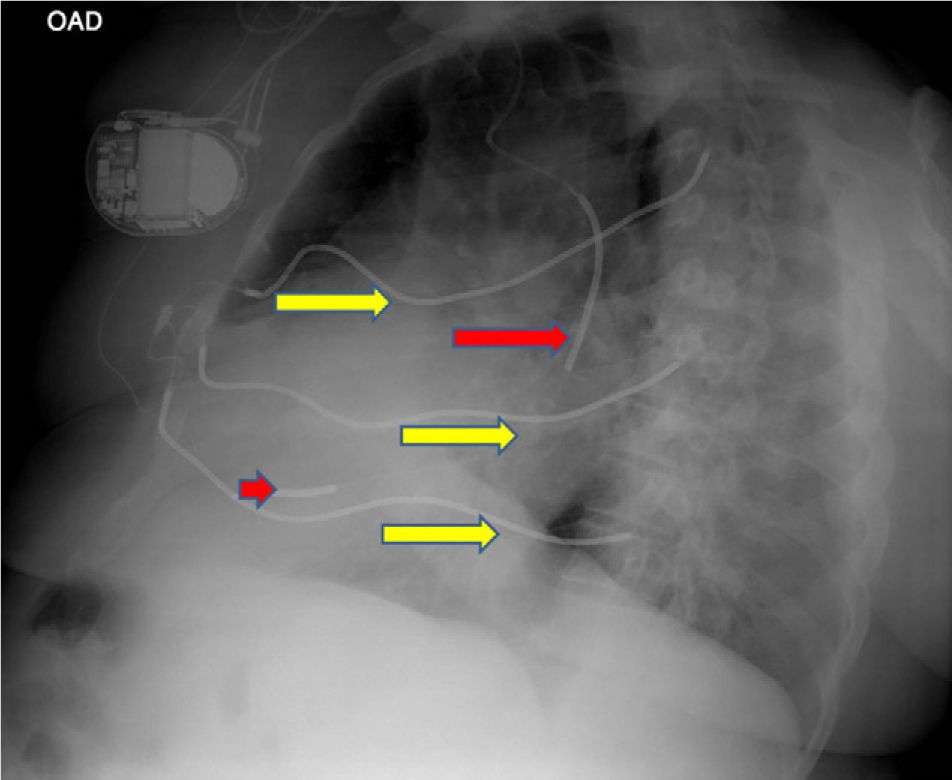
We present images from a patient who required implantation of an additional subcutaneous electrode array in the left hemithorax due to the device's failure to terminate VF during defibrillation testing, a situation that is now rarely encountered.
A 60-year-old male patient had a history of hypertension and extensive anterior myocardial infarction (MI) in 1997. Six years after the MI and following an episode of syncope, he was diagnosed with monomorphic ventricular tachycardia, with an R–R interval of 320ms. The echocardiogram revealed a dilated left ventricle with non-thickened walls, impaired global systolic function (ejection fraction of 35%), and apical dyskinesia, akinesia of the mid segments and hypokinesia of the basal segments of the anterior wall and anterior septum. Cardiac catheterization showed chronic occlusion of the proximal left anterior descending artery and 25% stenosis of the proximal circumflex. A Guidant® MINI II ICD was implanted for secondary prevention. High defibrillation thresholds were observed during ICD implantation, requiring implantation of an additional subcutaneous electrode array to enlarge the area of shock application (Figures 1–3).
In 2002, the patient underwent elective generator replacement due to battery depletion, a CPI Prizm 2 VR ICD being implanted, and defibrillation tests were successful with a 20-J shock.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cordeiro Piçarra, B. Cardiodesfibrilhador implantável com elétrodo de choque subcutâneo adicional. Rev Port Cardiol. 2012. doi:10.1016/j.repc.2012.02.006.